“自催化”的版本间的差异
小 (Moved page from wikipedia:en:Autocatalysis (history)) |
小 (Moved page from wikipedia:en:Autocatalysis (history)) |
||
| 第1行: | 第1行: | ||
| − | + | 此词条暂由彩云小译翻译,翻译字数共2466,未经人工整理和审校,带来阅读不便,请见谅。 | |
{{more citations needed|date=September 2010}} | {{more citations needed|date=September 2010}} | ||
| 第5行: | 第5行: | ||
A single [[chemical reaction]] is said to be '''autocatalytic''' if one of the reaction products is also a [[catalyst]] for the same or a coupled reaction.<ref name=Steinfeld>Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 1999) p.151-2 {{ISBN|0-13-737123-3}}</ref> Such a reaction is called an '''autocatalytic reaction'''. | A single [[chemical reaction]] is said to be '''autocatalytic''' if one of the reaction products is also a [[catalyst]] for the same or a coupled reaction.<ref name=Steinfeld>Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 1999) p.151-2 {{ISBN|0-13-737123-3}}</ref> Such a reaction is called an '''autocatalytic reaction'''. | ||
| − | A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction. | + | A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction. Such a reaction is called an autocatalytic reaction. |
| − | + | 一个单一的化学反应,如果其中一个反应产物也是同一反应或耦合反应的催化剂,则称为自催化反应。这种反应称为自催化反应。 | |
| − | |||
| − | |||
| − | |||
| − | |||
A ''set'' of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self-sustaining given an input of energy and food molecules (see [[autocatalytic set]]). | A ''set'' of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self-sustaining given an input of energy and food molecules (see [[autocatalytic set]]). | ||
| + | A set of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self-sustaining given an input of energy and food molecules (see autocatalytic set). | ||
| + | 如果一系列化学反应作为反应产物产生足够多的其他反应的催化剂,使整套化学反应在能量和食物分子输入的情况下能够自我维持,则可以说这些化学反应是”集体自我催化”的。 | ||
| − | |||
| − | |||
==Chemical reactions== | ==Chemical reactions== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{{main|Chemical reaction|Chemical kinetics}} | {{main|Chemical reaction|Chemical kinetics}} | ||
| 第39行: | 第27行: | ||
| − | + | A chemical reaction of two reactants and two products can be written as | |
| − | |||
| − | |||
A chemical reaction of two reactants and two products can be written as | A chemical reaction of two reactants and two products can be written as | ||
| + | 两种反应物和两种产物的化学反应可以写成 | ||
| − | |||
| − | |||
| − | |||
:<math> \alpha A + \beta B \rightleftharpoons \sigma S + \tau T</math> | :<math> \alpha A + \beta B \rightleftharpoons \sigma S + \tau T</math> | ||
| + | <math> \alpha A + \beta B \rightleftharpoons \sigma S + \tau T</math> | ||
| + | 阿尔法 a + 贝塔 b 右旋/西格玛 s + tau t | ||
| − | |||
| − | |||
where the Greek letters are [[stoichiometric coefficients]] and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily generalized to any number of reactants, products, and reactions. | where the Greek letters are [[stoichiometric coefficients]] and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily generalized to any number of reactants, products, and reactions. | ||
| + | |||
| + | where the Greek letters are stoichiometric coefficients and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily generalized to any number of reactants, products, and reactions. | ||
| + | |||
| + | 其中希腊字母是化学计量系数,大写拉丁字母代表化学物种。化学反应在正向和反向两个方向进行。这个方程可以很容易地推广到任意数量的反应物、产物和反应。 | ||
| 第69行: | 第57行: | ||
In [[chemical equilibrium]] the forward and reverse [[reaction rate]]s are such that each chemical species is being created at the same rate it is being destroyed. In other words, the rate of the forward reaction is equal to the rate of the reverse reaction. | In [[chemical equilibrium]] the forward and reverse [[reaction rate]]s are such that each chemical species is being created at the same rate it is being destroyed. In other words, the rate of the forward reaction is equal to the rate of the reverse reaction. | ||
| + | In chemical equilibrium the forward and reverse reaction rates are such that each chemical species is being created at the same rate it is being destroyed. In other words, the rate of the forward reaction is equal to the rate of the reverse reaction. | ||
| + | 在21世纪化学平衡,正向和反向反应的速率是如此之快,以至于每一种化学物质都以同样的速率被创造出来,同样的速率被摧毁。换句话说,前向反应的速率等于反向反应的速率。 | ||
| − | |||
| − | |||
:<math> k_+ [ A ]^\alpha [B ]^\beta = k_{-} [S ]^\sigma[T ]^\tau \,</math> | :<math> k_+ [ A ]^\alpha [B ]^\beta = k_{-} [S ]^\sigma[T ]^\tau \,</math> | ||
| + | <math> k_+ [ A ]^\alpha [B ]^\beta = k_{-} [S ]^\sigma[T ]^\tau \,</math> | ||
| + | K _ + [ a ] ^ alpha [ b ] ^ beta = k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math > | ||
| − | |||
| − | |||
Here, the brackets indicate the concentration of the chemical species, in [[mole (unit)|moles]] per liter, and k<sub>+</sub> and k<sub>−</sub> are [[rate constant]]s. | Here, the brackets indicate the concentration of the chemical species, in [[mole (unit)|moles]] per liter, and k<sub>+</sub> and k<sub>−</sub> are [[rate constant]]s. | ||
| + | Here, the brackets indicate the concentration of the chemical species, in moles per liter, and k<sub>+</sub> and k<sub>−</sub> are rate constants. | ||
| + | 这里,括号表示化学物质的浓度,以摩尔/升为单位,k < sub > + </sub > 和 k < sub >-</sub > 是速率常数。 | ||
| − | |||
| − | |||
===Far from equilibrium=== | ===Far from equilibrium=== | ||
| 第97行: | 第85行: | ||
Far from equilibrium, the forward and reverse reaction rates no longer balance and the concentration of reactants and products is no longer constant. For every forward reaction <math>\alpha </math> molecules of A are destroyed. For every reverse reaction <math>\alpha </math> molecules of A are created. In the case of an [[elementary reaction]] step the [[reaction order]] in each direction equals the molecularity, so that the rate of change in the number of moles of A is then | Far from equilibrium, the forward and reverse reaction rates no longer balance and the concentration of reactants and products is no longer constant. For every forward reaction <math>\alpha </math> molecules of A are destroyed. For every reverse reaction <math>\alpha </math> molecules of A are created. In the case of an [[elementary reaction]] step the [[reaction order]] in each direction equals the molecularity, so that the rate of change in the number of moles of A is then | ||
| + | Far from equilibrium, the forward and reverse reaction rates no longer balance and the concentration of reactants and products is no longer constant. For every forward reaction <math>\alpha </math> molecules of A are destroyed. For every reverse reaction <math>\alpha </math> molecules of A are created. In the case of an elementary reaction step the reaction order in each direction equals the molecularity, so that the rate of change in the number of moles of A is then | ||
| + | 远离平衡,正向和反向反应速率不再平衡,反应物和产物的浓度不再是恒定的。对于每一个前向反应,a 的分子都会被破坏。对于每一个逆反应,a 的分子都会被创造出来。在基本反应步骤中,每个方向的反应级数等于分子量,因此 a 的摩尔数的变化率就是 | ||
| − | |||
| − | |||
:<math>{d \over dt}[ A ] =-\alpha k_+ [ A ]^\alpha [B ]^\beta +\alpha k_{-} [S ]^\sigma[T ]^\tau \,</math> | :<math>{d \over dt}[ A ] =-\alpha k_+ [ A ]^\alpha [B ]^\beta +\alpha k_{-} [S ]^\sigma[T ]^\tau \,</math> | ||
| + | |||
| + | <math>{d \over dt}[ A ] =-\alpha k_+ [ A ]^\alpha [B ]^\beta +\alpha k_{-} [S ]^\sigma[T ]^\tau \,</math> | ||
| + | |||
| + | [ a ] =-alpha k _ + [ a ] ^ alpha [ b ] ^ beta + alpha k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math > | ||
:<math>{d \over dt}[ B ] =-\beta k_+ [ A ]^\alpha [B ]^\beta +\beta k_{-} [S ]^\sigma[T ]^\tau \,</math> | :<math>{d \over dt}[ B ] =-\beta k_+ [ A ]^\alpha [B ]^\beta +\beta k_{-} [S ]^\sigma[T ]^\tau \,</math> | ||
| − | < | + | <math>{d \over dt}[ B ] =-\beta k_+ [ A ]^\alpha [B ]^\beta +\beta k_{-} [S ]^\sigma[T ]^\tau \,</math> |
| − | + | [ b ] =-beta k _ + [ a ] ^ alpha [ b ] ^ beta + beta k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math > | |
:<math>{d \over dt}[ S ] =\sigma k_+ [ A ]^\alpha [B ]^\beta -\sigma k_{-} [S ]^\sigma[T ]^\tau \,</math> | :<math>{d \over dt}[ S ] =\sigma k_+ [ A ]^\alpha [B ]^\beta -\sigma k_{-} [S ]^\sigma[T ]^\tau \,</math> | ||
| + | |||
| + | <math>{d \over dt}[ S ] =\sigma k_+ [ A ]^\alpha [B ]^\beta -\sigma k_{-} [S ]^\sigma[T ]^\tau \,</math> | ||
| + | |||
| + | [ s ] = sigma k _ + [ a ] ^ alpha [ b ] ^ beta-sigma k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math > | ||
:<math>{d \over dt}[ T ] =\tau k_+ [ A ]^\alpha [B ]^\beta -\tau k_{-} [S ]^\sigma[T ]^\tau \,</math> | :<math>{d \over dt}[ T ] =\tau k_+ [ A ]^\alpha [B ]^\beta -\tau k_{-} [S ]^\sigma[T ]^\tau \,</math> | ||
| − | + | <math>{d \over dt}[ T ] =\tau k_+ [ A ]^\alpha [B ]^\beta -\tau k_{-} [S ]^\sigma[T ]^\tau \,</math> | |
| − | + | [ t ] = tau k _ + [ a ] ^ alpha [ b ] ^ beta-tau k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math > | |
This system of equations has a single stable [[Fixed point (mathematics)|fixed point]] when the forward rates and the reverse rates are equal (when <math>{d \over dt}=0</math> for every species). This means that the system evolves to the equilibrium state, and this is the only state to which it evolves.<ref>{{cite journal |last1=Ross |first1=John |last2=Garcia-Colin |first2=Leopoldo S. |title=Thermodynamics of chemical systems far from equilibrium |journal=The Journal of Physical Chemistry |date=March 1989 |volume=93 |issue=5 |pages=2091–2092 |doi=10.1021/j100342a075}}</ref> | This system of equations has a single stable [[Fixed point (mathematics)|fixed point]] when the forward rates and the reverse rates are equal (when <math>{d \over dt}=0</math> for every species). This means that the system evolves to the equilibrium state, and this is the only state to which it evolves.<ref>{{cite journal |last1=Ross |first1=John |last2=Garcia-Colin |first2=Leopoldo S. |title=Thermodynamics of chemical systems far from equilibrium |journal=The Journal of Physical Chemistry |date=March 1989 |volume=93 |issue=5 |pages=2091–2092 |doi=10.1021/j100342a075}}</ref> | ||
| + | |||
| + | This system of equations has a single stable fixed point when the forward rates and the reverse rates are equal (when <math>{d \over dt}=0</math> for every species). This means that the system evolves to the equilibrium state, and this is the only state to which it evolves. | ||
| + | |||
| + | 当正向速率和反向速率相等时,这个方程组有一个单一的稳定不动点(当每个物种的正向速率和反向速率相等时)。这意味着系统进化到平衡状态,而这是系统进化到的唯一状态。 | ||
| 第127行: | 第127行: | ||
===Autocatalytic reactions=== | ===Autocatalytic reactions=== | ||
| − | + | [[Image:Sigmoid curve for an autocatalytical reaction.jpg|256px|right|thumb|Sigmoid variation of product concentration in autocatalytic reactions]] | |
| − | + | Sigmoid variation of product concentration in autocatalytic reactions | |
| − | + | 自催化反应中产物浓度的 s 形变化 | |
Autocatalytic reactions are those in which at least one of the products is a reactant. Perhaps the simplest autocatalytic reaction can be written<ref name=Steinfeld/> | Autocatalytic reactions are those in which at least one of the products is a reactant. Perhaps the simplest autocatalytic reaction can be written<ref name=Steinfeld/> | ||
| + | |||
| + | Autocatalytic reactions are those in which at least one of the products is a reactant. Perhaps the simplest autocatalytic reaction can be written | ||
| + | |||
| + | 自催化反应是那些其中至少一个产物是反应物的反应。也许最简单的自催化反应可以写出来 | ||
:<math> A + B \rightleftharpoons 2B</math> | :<math> A + B \rightleftharpoons 2B</math> | ||
| + | |||
| + | <math>[A]=\frac{[A]_0+[B]_0}{1+\frac{[B]_0}{[A]_0}e^{([A]_0+[B]_0)kt}}</math> | ||
| + | |||
| + | < math > [ a ] = frac {[ a ] _ 0 + [ b ] _ 0}{1 + frac {[ b ] _ 0}{[ a ] _ 0} e ^ {(([ a ] _ 0 + [ b ] _ 0) kt }} </math > | ||
| − | + | and | |
| − | + | 及 | |
with the rate equations (for an elementary reaction) | with the rate equations (for an elementary reaction) | ||
| + | |||
| + | <math>[B]=\frac{[A]_0+[B]_0}{1+\frac{[A]_0}{[B]_0}e^{-([A]_0+[B]_0)kt}}</math>. | ||
| + | |||
| + | < math > [ b ] = frac {[ a ] _ 0 + [ b ] _ 0}{1 + frac {[ a ] _ 0}{[ b ] _ 0} e ^ {-([ a ] _ 0 + [ b ] _ 0) kt }} </math > . | ||
:<math>{d \over dt}[ A ] =- k_+ [ A ] [B ] + k_{-} [B ]^2 \,</math> | :<math>{d \over dt}[ A ] =- k_+ [ A ] [B ] + k_{-} [B ]^2 \,</math> | ||
| + | |||
| + | The graph for these equations is a sigmoid curve (specifically a logistic function), which is typical for autocatalytic reactions: these chemical reactions proceed slowly at the start (the induction period) because there is little catalyst present, the rate of reaction increases progressively as the reaction proceeds as the amount of catalyst increases and then it again slows down as the reactant concentration decreases. If the concentration of a reactant or product in an experiment follows a sigmoid curve, the reaction may be autocatalytic. | ||
| + | |||
| + | 这些方程的图表是一个 s 形曲线(特别是一个 Logistic函数) ,这是典型的自催化反应: 这些化学反应进行缓慢的开始(诱导期) ,因为有少量的催化剂存在,反应速度逐步增加,随着反应进行的催化剂数量增加,然后它再次减缓,作为反应物浓度降低。如果实验中反应物或产物的浓度符合 s 形曲线,则反应可能是自催化的。 | ||
:<math>{d \over dt}[ B ] = + k_+ [ A ] [B ] -k_{-} [B ]^2 \,</math>. | :<math>{d \over dt}[ B ] = + k_+ [ A ] [B ] -k_{-} [B ]^2 \,</math>. | ||
| 第151行: | 第167行: | ||
| − | + | These kinetic equations apply for example to the acid-catalyzed hydrolysis of some esters to carboxylic acids and alcohols. In the hurricane example, hurricanes are formed from unequal heating within the atmosphere. The Earth's atmosphere is then far from thermal equilibrium. The order of the Earth's atmosphere increases, but at the expense of the order of the sun. The sun is becoming more disorderly as it ages and throws off light and material to the rest of the universe. The total disorder of the sun and the earth increases despite the fact that orderly hurricanes are generated on earth. | |
| − | + | 这些动力学方程适用于某些酯类在酸催化下水解为羧酸和醇类。在飓风的例子中,飓风是由大气中不均匀的热量形成的。地球的大气层远离热平衡。地球大气层的次序增加了,但代价是太阳的次序。随着年龄的增长,太阳将光线和物质抛向宇宙的其他部分,它正变得越来越无序。尽管有序的飓风在地球上产生,但太阳和地球的总体混乱程度仍在增加。 | |
This reaction is one in which a molecule of species A interacts with a molecule of species B. The A molecule is converted into a B molecule. The final product consists of the original B molecule plus the B molecule created in the reaction. | This reaction is one in which a molecule of species A interacts with a molecule of species B. The A molecule is converted into a B molecule. The final product consists of the original B molecule plus the B molecule created in the reaction. | ||
| + | |||
| + | A similar example exists for living chemical systems. The sun provides energy to green plants. The green plants are food for other living chemical systems. The energy absorbed by plants and converted into chemical energy generates a system on earth that is orderly and far from chemical equilibrium. Here, the difference from chemical equilibrium is determined by an excess of reactants over the equilibrium amount. Once again, order on earth is generated at the expense of entropy increase of the sun. The total entropy of the earth and the rest of the universe increases, consistent with the Second Law. | ||
| + | |||
| + | 类似的例子也存在于活的化学系统中。太阳为绿色植物提供能量。绿色植物是其他生命化学系统的食物。植物吸收并转化为化学能的能量在地球上形成了一个有序的系统,这个系统远离化学平衡。在这里,反应物超过平衡量决定了化学平衡的差异。再一次,地球上的秩序是以太阳的熵增为代价而产生的。地球和宇宙其余部分的总熵增加,符合第二定律。 | ||
The key feature of these rate equations is that they are [[nonlinear]]; the second term on the right varies as the square of the concentration of B. This feature can lead to multiple fixed points of the system, much like a [[quadratic equation]] can have multiple roots. Multiple fixed points allow for multiple states of the system. A system existing in multiple [[macroscopic]] states is more orderly (has lower entropy) than a system in a single state. | The key feature of these rate equations is that they are [[nonlinear]]; the second term on the right varies as the square of the concentration of B. This feature can lead to multiple fixed points of the system, much like a [[quadratic equation]] can have multiple roots. Multiple fixed points allow for multiple states of the system. A system existing in multiple [[macroscopic]] states is more orderly (has lower entropy) than a system in a single state. | ||
| 第163行: | 第183行: | ||
| − | + | Some autocatalytic reactions also generate order in a system at the expense of its surroundings. For example, (clock reactions) have intermediates whose concentrations oscillate in time, corresponding to temporal order. Other reactions generate spatial separation of chemical species corresponding to spatial order. More complex reactions are involved in metabolic pathways and metabolic networks in biological systems. | |
| − | + | 一些自催化反应也以牺牲周围环境为代价,在系统中产生有序。例如,(时钟反应)有其浓度在时间上振荡的中间体,对应于时间顺序。其他反应产生空间分离的化学物质对应的空间秩序。更复杂的反应涉及到生物系统中的代谢途径和代谢网络。 | |
| − | + | The concentrations of A and B vary in time according to<ref name=Steinfeld/><ref name=Moore>Moore J.W. and [[Ralph Pearson|Pearson R.G.]] ''Kinetics and Mechanism'' (John Wiley 1981) p.26 {{ISBN|0-471-03558-0}}</ref> | |
:<math>[A]=\frac{[A]_0+[B]_0}{1+\frac{[B]_0}{[A]_0}e^{([A]_0+[B]_0)kt}}</math> | :<math>[A]=\frac{[A]_0+[B]_0}{1+\frac{[B]_0}{[A]_0}e^{([A]_0+[B]_0)kt}}</math> | ||
| − | and | + | The transition to order as the distance from equilibrium increases is not usually continuous. Order typically appears abruptly. The threshold between the disorder of chemical equilibrium and order is known as a phase transition. The conditions for a phase transition can be determined with the mathematical machinery of non-equilibrium thermodynamics. |
| − | + | 随着离平衡距离的增加,向有序的过渡通常是不连续的。秩序通常会突然出现。化学平衡和有序之间的无序阈值被称为相变。相变的条件可以用非平衡态热力学的数学方法来确定。 | |
| − | + | and | |
:<math>[B]=\frac{[A]_0+[B]_0}{1+\frac{[A]_0}{[B]_0}e^{-([A]_0+[B]_0)kt}}</math>. | :<math>[B]=\frac{[A]_0+[B]_0}{1+\frac{[A]_0}{[B]_0}e^{-([A]_0+[B]_0)kt}}</math>. | ||
| 第181行: | 第201行: | ||
The graph for these equations is a [[sigmoid function|sigmoid curve]] (specifically a [[logistic function]]), which is typical for autocatalytic reactions: these chemical reactions proceed slowly at the start (the [[induction period]]) because there is little catalyst present, the rate of reaction increases progressively as the reaction proceeds as the amount of catalyst increases and then it again slows down as the reactant concentration decreases. If the concentration of a reactant or product in an experiment follows a sigmoid curve, the reaction may be autocatalytic. | The graph for these equations is a [[sigmoid function|sigmoid curve]] (specifically a [[logistic function]]), which is typical for autocatalytic reactions: these chemical reactions proceed slowly at the start (the [[induction period]]) because there is little catalyst present, the rate of reaction increases progressively as the reaction proceeds as the amount of catalyst increases and then it again slows down as the reactant concentration decreases. If the concentration of a reactant or product in an experiment follows a sigmoid curve, the reaction may be autocatalytic. | ||
| − | + | A chemical reaction cannot oscillate about a position of final equilibrium because the second law of thermodynamics requires that a thermodynamic system approach equilibrium and not recede from it. For a closed system at constant temperature and pressure, the Gibbs free energy must decrease continuously and not oscillate. However it is possible that the concentrations of some reaction intermediates oscillate, and also that the rate of formation of products oscillates. | |
| − | + | 一个化学反应不能在一个最终平衡位置上振荡,因为热力学第二定律需要一个接近平衡的热力学系统,而不能退出平衡位置。对于一个温度和压力恒定的封闭系统,吉布斯自由能必须不断减小而不振荡。然而,一些反应中间体的浓度可能会振荡,产物的生成速率也可能振荡。 | |
| 第189行: | 第209行: | ||
These kinetic equations apply for example to the acid-catalyzed hydrolysis of some [[ester]]s to [[carboxylic acid]]s and [[alcohol]]s.<ref name=Moore/> There must be at least some acid present initially to start the catalyzed mechanism; if not the reaction must start by an alternate uncatalyzed path which is usually slower. The above equations for the catalyzed mechanism would imply that the concentration of acid product remains zero forever.<ref name=Moore/> | These kinetic equations apply for example to the acid-catalyzed hydrolysis of some [[ester]]s to [[carboxylic acid]]s and [[alcohol]]s.<ref name=Moore/> There must be at least some acid present initially to start the catalyzed mechanism; if not the reaction must start by an alternate uncatalyzed path which is usually slower. The above equations for the catalyzed mechanism would imply that the concentration of acid product remains zero forever.<ref name=Moore/> | ||
| − | |||
| − | |||
| + | The Lotka–Volterra equation is [[isomorphic with the predator–prey model and the two-reaction autocatalytic model. In this example baboons and cheetahs are equivalent to two different chemical species X and Y in autocatalytic reactions.]] | ||
| + | 方程[同构于捕食-食饵模型和双反应自催化模型。在这个例子中,狒狒和猎豹在自动催化反应中相当于两种不同的化学物种 x 和 y | ||
==Creation of order== | ==Creation of order== | ||
| − | + | Consider a coupled set of two autocatalytic reactions in which the concentration of one of the reactants A is much larger than its equilibrium value. In this case, the forward reaction rate is so much larger than the reverse rates that we can neglect the reverse rates. | |
| − | + | 考虑一组耦合的两个自催化反应,其中一个反应物 a 的浓度远远大于其平衡值。在这种情况下,正向反应速率远远大于反向反应速率,因此我们可以忽略反向反应速率。 | |
===Background=== | ===Background=== | ||
| + | |||
| + | <math> A + X \rightarrow 2X</math> | ||
| + | |||
| + | A + x 右行2X | ||
The [[second law of thermodynamics]] states that the disorder ([[entropy]]) of a physical or chemical system and its surroundings (a [[closed system]]) must increase with time. Systems left to themselves become increasingly [[random]], and orderly energy of a system like uniform motion degrades eventually to the random motion of particles in a [[heat bath]]. | The [[second law of thermodynamics]] states that the disorder ([[entropy]]) of a physical or chemical system and its surroundings (a [[closed system]]) must increase with time. Systems left to themselves become increasingly [[random]], and orderly energy of a system like uniform motion degrades eventually to the random motion of particles in a [[heat bath]]. | ||
| + | |||
| + | <math> X + Y \rightarrow 2Y</math> | ||
| + | |||
| + | (数学) x + y 右行2Y | ||
There are, however, many instances in which physical systems spontaneously become [[emergence|emergent]] or orderly. For example, despite the destruction they cause, [[hurricane]]s have a very orderly [[vortex]] motion when compared to the random motion of the air molecules in a closed room. Even more spectacular is the order created by chemical systems; the most dramatic being the order associated with life. | There are, however, many instances in which physical systems spontaneously become [[emergence|emergent]] or orderly. For example, despite the destruction they cause, [[hurricane]]s have a very orderly [[vortex]] motion when compared to the random motion of the air molecules in a closed room. Even more spectacular is the order created by chemical systems; the most dramatic being the order associated with life. | ||
| + | |||
| + | <math> Y \rightarrow E</math> | ||
| + | |||
| + | [数学,数学] | ||
This is consistent with the Second Law, which requires that the total disorder of a system ''and its surroundings'' must increase with time. Order can be created in a system by an even greater decrease in order of the system's surroundings.<ref>{{cite book | author=Ilya Prigogine | title=From Being to Becoming: Time and Complexity in the Physical Sciences | location=San Francisco | publisher=W. H. Freeman | year=1980 | isbn=978-0-7167-1107-0 | author-link=Ilya Prigogine | url-access=registration | url=https://archive.org/details/frombeingtobecom00ipri }} | This is consistent with the Second Law, which requires that the total disorder of a system ''and its surroundings'' must increase with time. Order can be created in a system by an even greater decrease in order of the system's surroundings.<ref>{{cite book | author=Ilya Prigogine | title=From Being to Becoming: Time and Complexity in the Physical Sciences | location=San Francisco | publisher=W. H. Freeman | year=1980 | isbn=978-0-7167-1107-0 | author-link=Ilya Prigogine | url-access=registration | url=https://archive.org/details/frombeingtobecom00ipri }} | ||
| + | |||
| + | with the rate equations | ||
| + | |||
| + | 用速率方程式 | ||
</ref> In the hurricane example, hurricanes are formed from unequal heating within the atmosphere. The Earth's atmosphere is then far from [[thermal equilibrium]]. The order of the Earth's atmosphere increases, but at the expense of the order of the sun. The sun is becoming more disorderly as it ages and throws off light and material to the rest of the universe. The total disorder of the sun and the earth increases despite the fact that orderly hurricanes are generated on earth. | </ref> In the hurricane example, hurricanes are formed from unequal heating within the atmosphere. The Earth's atmosphere is then far from [[thermal equilibrium]]. The order of the Earth's atmosphere increases, but at the expense of the order of the sun. The sun is becoming more disorderly as it ages and throws off light and material to the rest of the universe. The total disorder of the sun and the earth increases despite the fact that orderly hurricanes are generated on earth. | ||
| + | |||
| + | <math>{d \over dt}[ X ] = k_1 [ A ] [X ] - k_{2} [X ][Y ] \,</math> | ||
| + | |||
| + | < math > { d over dt }[ x ] = k_1[ a ][ x ]-k_2}[ x ][ y ] ,</math > | ||
A similar example exists for living chemical systems. The sun provides energy to green plants. The green plants are food for other living chemical systems. The energy absorbed by plants and converted into chemical energy generates a system on earth that is orderly and far from [[chemical equilibrium]]. Here, the difference from chemical equilibrium is determined by an excess of reactants over the equilibrium amount. Once again, order on earth is generated at the expense of entropy increase of the sun. The total entropy of the earth and the rest of the universe increases, consistent with the Second Law. | A similar example exists for living chemical systems. The sun provides energy to green plants. The green plants are food for other living chemical systems. The energy absorbed by plants and converted into chemical energy generates a system on earth that is orderly and far from [[chemical equilibrium]]. Here, the difference from chemical equilibrium is determined by an excess of reactants over the equilibrium amount. Once again, order on earth is generated at the expense of entropy increase of the sun. The total entropy of the earth and the rest of the universe increases, consistent with the Second Law. | ||
| + | |||
| + | <math>{d \over dt}[ Y ] = k_2 [ X ] [Y ] - k_{3} [Y ] \,</math>. | ||
| + | |||
| + | [ y ] = k _ 2[ x ][ y ]-k _ {3}[ y ] ,[/math ]. | ||
Some autocatalytic reactions also generate order in a system at the expense of its surroundings. For example, ([[clock reactions]]) have [[reaction intermediate|intermediates]] whose concentrations oscillate in time, corresponding to temporal order. Other reactions generate spatial separation of [[chemical species]] corresponding to spatial order. More complex reactions are involved in [[metabolic pathway]]s and [[metabolic network]]s in [[biological systems]]. | Some autocatalytic reactions also generate order in a system at the expense of its surroundings. For example, ([[clock reactions]]) have [[reaction intermediate|intermediates]] whose concentrations oscillate in time, corresponding to temporal order. Other reactions generate spatial separation of [[chemical species]] corresponding to spatial order. More complex reactions are involved in [[metabolic pathway]]s and [[metabolic network]]s in [[biological systems]]. | ||
| + | |||
| + | Here, we have neglected the depletion of the reactant A, since its concentration is so large. The rate constants for the three reactions are <math>k_1</math>, <math>k_2</math>, and <math>k_3</math>, respectively. | ||
| + | |||
| + | 在这里,我们忽略了反应物 a 的耗尽,因为它的浓度很大。这三个反应的速率常数分别是 < math > k _ 1 </math > ,< math > k _ 2 </math > ,和 < math > k _ 3 </math > 。 | ||
The transition to order as the distance from equilibrium increases is not usually continuous. Order typically appears abruptly. The threshold between the disorder of chemical equilibrium and order is known as a [[phase transition]]. The conditions for a phase transition can be determined with the mathematical machinery of [[non-equilibrium thermodynamics]]. | The transition to order as the distance from equilibrium increases is not usually continuous. Order typically appears abruptly. The threshold between the disorder of chemical equilibrium and order is known as a [[phase transition]]. The conditions for a phase transition can be determined with the mathematical machinery of [[non-equilibrium thermodynamics]]. | ||
| + | |||
| + | This system of rate equations is known as the Lotka–Volterra equation and is most closely associated with population dynamics in predator–prey relationships. This system of equations can yield oscillating concentrations of the reaction intermediates X and Y. The amplitude of the oscillations depends on the concentration of A (which decreases without oscillation). Such oscillations are a form of emergent temporal order that is not present in equilibrium. | ||
| + | |||
| + | 这个速率方程组被称为 Lotka-Volterra 方程,在捕食者-食饵关系中与族群动态最密切相关。这个方程组可以产生反应中间体 x 和 y 的振荡浓度。振荡的振幅取决于 a 的浓度(a 的浓度下降而没有振荡)。这种振荡是一种涌现的时间顺序,在平衡中不存在。 | ||
===Temporal order=== | ===Temporal order=== | ||
| 第240行: | 第292行: | ||
====Idealized example: Lotka–Volterra equation==== | ====Idealized example: Lotka–Volterra equation==== | ||
| + | |||
| + | Another example of a system that demonstrates temporal order is the Brusselator (see Prigogine reference). It is characterized by the reactions | ||
| + | |||
| + | 另一个演示时间顺序的系统例子是 Brusselator (见 Prigogine 参考文献)。人们的反应是拥有属性的 | ||
[[File:CentralTendencyLV.jpg|thumb|right|350px|The Lotka–Volterra equation is [[isomorphic]] with the predator–prey model and the two-reaction autocatalytic model. In this example baboons and cheetahs are equivalent to two different chemical species X and Y in autocatalytic reactions.]] | [[File:CentralTendencyLV.jpg|thumb|right|350px|The Lotka–Volterra equation is [[isomorphic]] with the predator–prey model and the two-reaction autocatalytic model. In this example baboons and cheetahs are equivalent to two different chemical species X and Y in autocatalytic reactions.]] | ||
Consider a coupled set of two autocatalytic reactions in which the concentration of one of the reactants A is much larger than its equilibrium value. In this case, the forward reaction rate is so much larger than the reverse rates that we can neglect the reverse rates. | Consider a coupled set of two autocatalytic reactions in which the concentration of one of the reactants A is much larger than its equilibrium value. In this case, the forward reaction rate is so much larger than the reverse rates that we can neglect the reverse rates. | ||
| + | |||
| + | <math> A \rightarrow X</math> | ||
| + | |||
| + | 《数学》 a right tarrow x | ||
:<math> A + X \rightarrow 2X</math> | :<math> A + X \rightarrow 2X</math> | ||
| + | |||
| + | <math> 2X + Y \rightarrow 3X</math> | ||
| + | |||
| + | 2X + y 右行3X | ||
:<math> X + Y \rightarrow 2Y</math> | :<math> X + Y \rightarrow 2Y</math> | ||
| + | |||
| + | <math> B + X \rightarrow Y + D</math> | ||
| + | |||
| + | B + x 右边 y + d | ||
:<math> Y \rightarrow E</math> | :<math> Y \rightarrow E</math> | ||
| + | |||
| + | <math> X \rightarrow E</math> | ||
| + | |||
| + | X 右边的 e | ||
| + | |||
| + | |||
| + | |||
| + | with the rate equations | ||
| + | |||
| + | with the rate equations | ||
| + | |||
| + | 用速率方程式 | ||
| + | |||
| + | |||
| + | |||
| + | :<math>{d \over dt}[ X ] = k_1 [ A ] [X ] - k_{2} [X ][Y ] \,</math> | ||
| + | |||
| + | <math>{d \over dt}[ X ] = [A ] + [ X ]^2 [Y ] - [B ] [X ] - [X ] \,</math> | ||
| + | |||
| + | < math > { d over dt }[ x ] = [ a ] + [ x ] ^ 2[ y ]-[ b ][ x ]-[ x ] ,</math > | ||
| + | |||
| + | |||
| + | |||
| + | :<math>{d \over dt}[ Y ] = k_2 [ X ] [Y ] - k_{3} [Y ] \,</math>. | ||
| + | |||
| + | <math>{d \over dt}[ Y ] = [B ] [X ] - [ X ]^2 [Y ] \,</math> | ||
| + | |||
| + | [数学]{ d/dt }[ y ] = [ b ][ x ]-[ x ] ^ 2[ y ] ,[/math ] | ||
| + | |||
| + | |||
| + | |||
| + | Here, we have neglected the depletion of the reactant A, since its concentration is so large. The rate constants for the three reactions are <math>k_1</math>, <math>k_2</math>, and <math>k_3</math>, respectively. | ||
| + | |||
| + | where, for convenience, the rate constants have been set to 1. | ||
| + | |||
| + | 为了方便起见,速率常数设置为1。 | ||
| + | |||
| + | |||
| + | |||
| + | This system of rate equations is known as the [[Lotka–Volterra equation]] and is most closely associated with [[population dynamics]] in predator–prey relationships. This system of equations can yield oscillating concentrations of the reaction intermediates X and Y. The amplitude of the oscillations depends on the concentration of A (which decreases without oscillation). Such oscillations are a form of emergent temporal order that is not present in equilibrium. | ||
| + | |||
| + | The Brusselator in the unstable regime. A=1. B=2.5. X(0)=1. Y(0)=0. The system approaches a [[limit cycle. For B<1+A the system is stable and approaches a fixed point.]] | ||
| + | |||
| + | 不稳定政权中的布鲁塞尔人。1.2.5.X (0) = 1.Y (0) = 0.该系统接近[极限环]。对于 b < 1 + a,系统是稳定的,并且接近一个固定点。] | ||
| + | |||
| + | |||
| + | |||
| + | The Brusselator has a fixed point at | ||
| + | |||
| + | 布鲁塞尔终结者有一个固定点 | ||
| + | |||
| + | ====Another idealized example: Brusselator==== | ||
| + | |||
| + | |||
| + | |||
| + | <math>[ X ] = A \,</math> | ||
| + | |||
| + | [数学] = a,[数学] | ||
| + | |||
| + | Another example of a system that demonstrates temporal order is the [[Brusselator]] (see Prigogine reference). It is characterized by the reactions | ||
| + | |||
| + | |||
| + | |||
| + | <math>[ Y ] = {B \over A} \,</math>. | ||
| + | |||
| + | [ math ][ y ] = { b over a } ,[ math ]. | ||
| + | |||
| + | :<math> A \rightarrow X</math> | ||
| + | |||
| + | |||
| + | |||
| + | The fixed point becomes unstable when | ||
| + | |||
| + | 定点变得不稳定,当 | ||
| + | |||
| + | :<math> 2X + Y \rightarrow 3X</math> | ||
| + | |||
| + | |||
| + | |||
| + | <math> B>1+A^2 \,</math> | ||
| + | |||
| + | 1 + a ^ 2, | ||
| + | |||
| + | :<math> B + X \rightarrow Y + D</math> | ||
| + | |||
| + | |||
| + | |||
| + | leading to an oscillation of the system. Unlike the Lotka-Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a limit cycle. | ||
| + | |||
| + | 导致了系统的振荡。与洛特卡-沃尔泰拉方程不同,布鲁塞尔振荡器的振荡并不取决于最初反应物的数量。相反,在足够的时间之后,振荡接近极限环。 | ||
| + | |||
| + | :<math> X \rightarrow E</math> | ||
| + | |||
| + | |||
| + | |||
| + | with the rate equations | ||
| + | |||
| + | An idealized example of spatial spontaneous symmetry breaking is the case in which we have two boxes of material separated by a permeable membrane so that material can diffuse between the two boxes. It is assumed that identical Brusselators are in each box with nearly identical initial conditions. (see Prigogine reference) | ||
| + | |||
| + | 一个理想化的空间自发对称性破缺的例子是,我们有两个盒子的材料被一个可渗透的薄膜分开,这样材料可以在两个盒子之间扩散。我们假设在每个盒子中都有相同的布鲁塞尔子,并且具有几乎相同的初始条件。(见 Prigogine 参考文献) | ||
| + | |||
| + | |||
| + | |||
| + | :<math>{d \over dt}[ X ] = [A ] + [ X ]^2 [Y ] - [B ] [X ] - [X ] \,</math> | ||
| + | |||
| + | <math>{d \over dt}[ X_1 ] = [A ] + [ X _1]^2 [Y_1 ] - [B ] [X_1 ] - [X_1 ] + D_x\left( X_2 - X_1 \right)\,</math> | ||
| + | |||
| + | < math > { d over dt }[ x _ 1] = [ a ] + [ x _ 1] ^ 2[ y _ 1]-[ b ][ x _ 1]-[ x _ 1] + d _ x 左(x _ 2-x _ 1右) ,</math > | ||
| + | |||
| + | |||
| + | |||
| + | :<math>{d \over dt}[ Y ] = [B ] [X ] - [ X ]^2 [Y ] \,</math> | ||
| + | |||
| + | <math>{d \over dt}[ Y_1 ] = [B ] [X_1 ] - [ X_1 ]^2 [Y_1 ] + D_y\left( Y_2 - Y_1\right) \,</math> | ||
| + | |||
| + | < math > { d over dt }[ y _ 1] = [ b ][ x _ 1]-[ x _ 1] ^ 2[ y _ 1] + d _ y 左(y _ 2-y _ 1右) ,</math > | ||
| + | |||
| + | |||
| + | |||
| + | where, for convenience, the rate constants have been set to 1. | ||
| + | |||
| + | <math>{d \over dt}[ X_2 ] = [A ] + [ X _2]^2 [Y_2 ] - [B ] [X_2 ] - [X_2 ] + D_x\left( X_1 - X_2 \right)\,</math> | ||
| + | |||
| + | [ x _ 2] = [ a ] + [ x _ 2] ^ 2[ y _ 2]-[ b ][ x _ 2]-[ x _ 2] + d _ x 左(x _ 1-x _ 2右) ,</math > | ||
| + | |||
| + | |||
| + | |||
| + | [[File:080205 Brusselator picture.jpg|thumb|right|350px|The Brusselator in the unstable regime. A=1. B=2.5. X(0)=1. Y(0)=0. The system approaches a [[limit cycle]]. For B<1+A the system is stable and approaches a [[Fixed point (mathematics)|fixed point]].]] | ||
| + | |||
| + | <math>{d \over dt}[ Y_2 ] = [B ] [X_2 ] - [ X_2 ]^2 [Y_2 ] + D_y\left( Y_1 - Y_2\right) \,</math> | ||
| + | |||
| + | [ math ]{ d over dt }[ y _ 2] = [ b ][ x _ 2]-[ x _ 2] ^ 2[ y _ 2] + d _ y 左(y _ 1-y _ 2右) ,</math > | ||
| + | |||
| + | The Brusselator has a fixed point at | ||
| + | |||
| + | |||
| + | |||
| + | Here, the numerical subscripts indicate which box the material is in. There are additional terms proportional to the diffusion coefficient D that account for the exchange of material between boxes. | ||
| + | |||
| + | 在这里,数字下标表示材料在哪个盒子里。还有一些与扩散系数 d 成比例的附加项,用于解释盒子之间的物质交换。 | ||
| + | |||
| + | :<math>[ X ] = A \,</math> | ||
| + | |||
| + | |||
| + | |||
| + | If the system is initiated with the same conditions in each box, then a small fluctuation will lead to separation of materials between the two boxes. One box will have a predominance of X, and the other will have a predominance of Y. | ||
| + | |||
| + | 如果系统启动时每个箱子的条件相同,那么一个小的波动将导致两个箱子之间的物料分离。一个盒子将具有 x 的优势,而另一个盒子将具有 y 的优势。 | ||
| + | |||
| + | :<math>[ Y ] = {B \over A} \,</math>. | ||
| + | |||
| + | |||
| + | |||
| + | The fixed point becomes unstable when | ||
| + | |||
| + | |||
| + | |||
| + | Real examples of clock reactions are the Belousov–Zhabotinsky reaction (BZ reaction), the Briggs–Rauscher reaction, the Bray–Liebhafsky reaction and the iodine clock reaction. These are oscillatory reactions, and the concentration of products and reactants can be approximated in terms of damped oscillations. | ||
| + | |||
| + | 时钟反应的真实例子是 Belousov-Zhabotinsky 反应(BZ 反应) ,Briggs-Rauscher 反应,Bray-Liebhafsky 反应和碘钟反应。这些都是振荡反应,产物和反应物的浓度可以用阻尼振荡来近似。 | ||
| + | |||
| + | :<math> B>1+A^2 \,</math> | ||
| + | |||
| + | |||
| + | |||
| + | The best-known reaction, the BZ reaction, can be created with a mixture of potassium bromate <chem>(KBrO3)</chem>, malonic acid <chem>(CH2(COOH)2)</chem>, and manganese sulfate <chem>(MnSO4)</chem> prepared in a heated solution with sulfuric acid <chem>(H2SO4)</chem> as solvent. | ||
| + | |||
| + | 其中最著名的反应是 BZ 反应,它是在硫酸 </chem > (H2SO4) </chem > (H2SO4) </chem > (CH2(COOH)2) </chem > 和用硫酸 </chem > (MnSO4) </chem > 加热溶液中制得的混合溴酸钾。 | ||
| + | |||
| + | leading to an oscillation of the system. Unlike the Lotka-Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a [[limit cycle]].<ref>{{cite web | ||
| + | |||
| + | |url=http://www.math.ohio-state.edu/~ault/Papers/Brusselator.pdf | ||
| + | |||
| + | |title=Archived copy | ||
| + | |||
| + | Another autocatalytic system is one driven by light coupled to photo-polymerization reactions. In a process termed optical autocatalysis, positive feedback is created between light intensity and photo-polymerization rate, via polymerization-induced increases in the refractive index. Light's preference to occupy regions of higher refractive index results in leakage of light into regions of higher molecular weight, thereby amplifying the photo-chemical reaction. The positive feedback may be expressed as: | ||
| + | |||
| + | 另一种自催化体系是由光与光聚合反应耦合驱动的体系。在一个被称为光学自催化的过程中,光强度和光聚合速率之间产生正反馈,通过聚合诱导增加折射率。光喜欢占据折射率较高的区域,导致光线泄漏到分子量较高的区域,从而放大了光化学反应。积极的反馈可以表示为: | ||
| + | |||
| + | |accessdate=2015-10-15 | ||
| + | |||
| + | |url-status=dead | ||
| + | |||
| + | <math>\text{polymerization rate} \to \text{molecular weight}/\text{refractive index} \to \text{intensity}</math> | ||
| + | |||
| + | 文本{分子量}/文本{折射率}到文本{密度} | ||
| + | |||
| + | |archiveurl=https://web.archive.org/web/20081217020941/http://www.math.ohio-state.edu/~ault/Papers/Brusselator.pdf | ||
| + | |||
| + | |archivedate=2008-12-17 | ||
| + | |||
| + | Noting that photo-polymerization rate is proportional to intensity and that refractive index is proportional to molecular weight, the positive feedback between intensity and photo-polymerization establishes the auto-catalytic behavior. Optical auto-catalysis has been shown to result on spontaneous pattern formation in photopolymers. Hosein and co-workers discovered that optical autocatalysis can also occur in photoreactive polymer blends, and that the process can induce binary phase morphologies with the same pattern as the light profile. Glycolysis consists of the degradation of one molecule of glucose and the overall production of two molecules of ATP. The process is therefore of great importance to the energetics of living cells. The global glycolysis reaction involves glucose, ADP, NAD, pyruvate, ATP, and NADH. | ||
| + | |||
| + | 注意到光聚合速率与强度成正比,折射率与分子量成正比,强度与光聚合之间的正反馈建立了自催化行为。光学自催化已被证明是光聚合物自发形成图案的原因。Hosein 和他的合作者发现,光学自催化也可以发生在光反应聚合物共混物中,这一过程可以诱导形成与光型相同的二元相形态。糖酵解是由一个葡萄糖分子的降解和两个 ATP 分子的全部产生组成。因此,这个过程对活细胞的能量学来说是非常重要的。糖酵解反应包括葡萄糖、 ADP、 NAD、丙酮酸、 ATP 和 NADH。 | ||
| + | |||
| + | }} Dynamics of the Brusselator | ||
| + | |||
| + | </ref> | ||
| + | |||
| + | <chem>glucose{} + 2ADP{} + 2P_\mathit{i}{} + 2NAD -> 2(pyruvate){} + 2ATP{} + 2NADH</chem>. | ||
| + | |||
| + | < chem > glucose {} + 2ADP {} + 2P _ mathit { i }{} + 2NAD-> 2(丙酮酸盐){} + 2ATP {} + 2NADH </chem > 。 | ||
| + | |||
| + | |||
| + | |||
| + | ===Spatial order=== | ||
| + | |||
| + | The details of the process are quite involved, however, a section of the process is autocatalyzed by phosphofructokinase (PFK). This portion of the process is responsible for oscillations in the pathway that lead to the process oscillating between an active and an inactive form. Thus, the autocatalytic reaction can modulate the process. | ||
| + | |||
| + | 该过程的细节相当复杂,然而,该过程的一部分是由磷酸果糖激酶(PFK)自催化的。这部分的过程负责振荡的路径,导致过程之间的振荡活跃和非活跃的形式。因此,自催化反应可以调节这一过程。 | ||
| + | |||
| + | An idealized example of spatial [[spontaneous symmetry breaking]] is the case in which we have two boxes of material separated by a permeable membrane so that material can [[diffusion|diffuse]] between the two boxes. It is assumed that identical Brusselators are in each box with nearly identical initial conditions. (see Prigogine reference) | ||
| + | |||
| + | |||
| + | |||
| + | :<math>{d \over dt}[ X_1 ] = [A ] + [ X _1]^2 [Y_1 ] - [B ] [X_1 ] - [X_1 ] + D_x\left( X_2 - X_1 \right)\,</math> | ||
| + | |||
| + | |||
| + | |||
| + | It is possible to use the results from an autocatalytic reaction coupled with reaction–diffusion system theory to tailor the design of a thin layer. The autocatalytic process allows controlling the nonlinear behavior of the oxidation front, which is used to establish the initial geometry needed to generate the arbitrary final geometry. It has been successfully done in the wet oxidation of <math>Al_xGa_{1-x}As</math> to obtain arbitrary shaped layers of <math>AlO_x</math>. | ||
| + | |||
| + | 利用自催化反应结合反应扩散系统理论的结果来设计薄层是可能的。自催化过程允许控制氧化前沿的非线性行为,用于建立生成任意最终几何所需的初始几何。在湿式氧化法中,成功地获得了任意形状的氧化铝层。 | ||
| + | |||
| + | :<math>{d \over dt}[ Y_1 ] = [B ] [X_1 ] - [ X_1 ]^2 [Y_1 ] + D_y\left( Y_2 - Y_1\right) \,</math> | ||
| + | |||
| + | |||
| + | |||
| + | :<math>{d \over dt}[ X_2 ] = [A ] + [ X _2]^2 [Y_2 ] - [B ] [X_2 ] - [X_2 ] + D_x\left( X_1 - X_2 \right)\,</math> | ||
| + | |||
| + | |||
| + | |||
| + | The initial amounts of reactants determine the distance from a chemical equilibrium of the system. The greater the initial concentrations the further the system is from equilibrium. As the initial concentration increases, an abrupt change in order occurs. This abrupt change is known as phase transition. At the phase transition, fluctuations in macroscopic quantities, such as chemical concentrations, increase as the system oscillates between the more ordered state (lower entropy, such as ice) and the more disordered state (higher entropy, such as liquid water). Also, at the phase transition, macroscopic equations, such as the rate equations, fail. Rate equations can be derived from microscopic considerations. The derivations typically rely on a mean field theory approximation to microscopic dynamical equations. Mean field theory breaks down in the presence of large fluctuations (see Mean field theory article for a discussion). Therefore, since large fluctuations occur in the neighborhood of a phase transition, macroscopic equations, such as rate equations, fail. As the initial concentration increases further, the system settles into an ordered state in which fluctuations are again small. (see Prigogine reference) | ||
| + | |||
| + | 反应物的初始数量决定了反应体系到化学平衡的距离。初始浓度越大,系统离平衡越远。随着初始浓度的增加,有序发生突变。这种突然的变化称为相变。在相变过程中,宏观量(如化学浓度)的涨落会随着系统在更有序的状态(如冰)和更无序的状态(如液态水)之间振荡而增加。此外,在相变时,宏观方程,如速率方程,失败。速率方程可以从微观考虑推导出来。推导通常依赖于平均场理论对微观动力学方程的近似。在存在大的波动时,平均场理论崩溃了(见平均场理论文章的讨论)。因此,由于大的波动发生在附近的相变,宏观方程,如速率方程,失败。随着初始浓度的进一步增加,系统进入有序状态,波动再次变小。(见 Prigogine 参考文献) | ||
| + | |||
| + | :<math>{d \over dt}[ Y_2 ] = [B ] [X_2 ] - [ X_2 ]^2 [Y_2 ] + D_y\left( Y_1 - Y_2\right) \,</math> | ||
| + | |||
| + | |||
| + | |||
| + | Here, the numerical subscripts indicate which box the material is in. There are additional terms proportional to the diffusion coefficient D that account for the exchange of material between boxes. | ||
| + | |||
| + | |||
| + | |||
| + | Asymmetric autocatalysis occurs when the reaction product is chiral and thus acts as a chiral catalyst for its own production. Reactions of this type, such as the Soai reaction, have the property that they can amplify a very small enantiomeric excess into a large one. This has been proposed as an important step in the origin of biological homochirality. | ||
| + | |||
| + | 不对称自催化反应发生在反应产物是手性的情况下,因此可以作为手性催化剂自身生产。这种类型的反应,比如 Soai 反应,具有将非常小的对映体过量百分数放大成大的反应的特性。这被认为是生物同手性起源的重要步骤。 | ||
| + | |||
| + | If the system is initiated with the same conditions in each box, then a small fluctuation will lead to separation of materials between the two boxes. One box will have a predominance of X, and the other will have a predominance of Y. | ||
| + | |||
| + | |||
| + | |||
| + | ==Real examples== | ||
| + | |||
| + | |||
| + | |||
| + | Real examples of [[clock reaction]]s are the [[Belousov–Zhabotinsky reaction]] (BZ reaction), the [[Briggs–Rauscher reaction]], the [[Bray–Liebhafsky reaction]] and the [[iodine clock reaction]]. These are oscillatory reactions, and the concentration of products and reactants can be approximated in terms of [[damping|damped]] [[oscillation]]s. | ||
| + | |||
| + | In 1995 Stuart Kauffman proposed that life initially arose as autocatalytic chemical networks. | ||
| + | |||
| + | 1995年,斯图尔特 · 考夫曼提出生命最初是以自催化化学网络的形式出现的。 | ||
| + | |||
| + | |||
| + | |||
| + | The best-known reaction, the BZ reaction, can be created with a mixture of potassium bromate <chem>(KBrO3)</chem>, malonic acid <chem>(CH2(COOH)2)</chem>, and manganese sulfate <chem>(MnSO4)</chem> prepared in a heated solution with sulfuric acid <chem>(H2SO4)</chem> as solvent.<ref>{{Cite web|url=http://online.redwoods.cc.ca.us/instruct/darnold/deproj/Sp98/Gabe/|title=The Belousov-Zhabotinsky Reaction|last=Peterson|first=Gabriel|date=|website=|accessdate=|archiveurl=https://web.archive.org/web/20121231011212/http://online.redwoods.cc.ca.us/instruct/darnold/deproj/Sp98/Gabe/|archivedate=December 31, 2012}}</ref> | ||
| + | |||
| + | British ethologist Richard Dawkins wrote about autocatalysis as a potential explanation for abiogenesis in his 2004 book The Ancestor's Tale. He cites experiments performed by Julius Rebek and his colleagues at the Scripps Research Institute in California in which they combined amino adenosine and pentafluorophenyl ester with the autocatalyst amino adenosine triacid ester (AATE). One system from the experiment contained variants of AATE which catalyzed the synthesis of themselves. This experiment demonstrated the possibility that autocatalysts could exhibit competition within a population of entities with heredity, which could be interpreted as a rudimentary form of natural selection, and that certain environmental changes (such as irradiation) could alter the chemical structure of some of these self-replicating molecules (an analog for mutation) in such ways that could either boost or interfere with its ability to react, thus boosting or interfering with its ability to replicate and spread in the population. | ||
| + | |||
| + | 英国动物行为学家理查德 · 道金斯在他2004年出版的《祖先的故事》一书中提到了自我催化作为自然发生的潜在解释。他引用了 Julius Rebek 和他的同事们在加利福尼亚斯克里普斯研究所进行的实验,他们将氨基腺苷和五氟苯酯与氨基腺苷三酸酯(AATE)结合在一起。实验中的一个系统包含了催化自身合成的 AATE 的变体。这项实验证明了这样一种可能性,即自动催化剂可以在具有遗传性的实体群体中展现竞争,这可以被解释为一种基本的自然选择形式,而且某些环境变化(如辐照)可以改变某些自我复制分子(变异的类似物)的化学结构,这种方式可以增强或干扰其反应能力,从而增强或干扰其复制和在群体中传播的能力。 | ||
| + | |||
| + | |||
| + | |||
| + | == Optics example == | ||
| + | |||
| + | Autocatalysis plays a major role in the processes of life. Two researchers who have emphasized its role in the origins of life are Robert Ulanowicz and Stuart Kauffman. | ||
| + | |||
| + | 自动催化在生命过程中起着重要的作用。强调罗伯特·尤兰维奇在生命起源中的作用的两位研究人员是斯图尔特 · 考夫曼和斯图尔特 · 考夫曼。 | ||
| + | |||
| + | Another autocatalytic system is one driven by light coupled to photo-polymerization reactions. In a process termed optical autocatalysis, positive feedback is created between light intensity and photo-polymerization rate, via polymerization-induced increases in the refractive index. Light's preference to occupy regions of higher refractive index results in leakage of light into regions of higher molecular weight, thereby amplifying the photo-chemical reaction. The positive feedback may be expressed as:<ref name=":0">{{Cite journal|last=Biria|first=Saeid|last2=Malley|first2=Phillip P. A.|last3=Kahan|first3=Tara F.|last4=Hosein|first4=Ian D.|date=2016-11-15|title=Optical Autocatalysis Establishes Novel Spatial Dynamics in Phase Separation of Polymer Blends during Photocuring|journal=ACS Macro Letters|volume=5|issue=11|pages=1237–1241|doi=10.1021/acsmacrolett.6b00659}}</ref> | ||
| + | |||
| + | |||
| + | |||
| + | Autocatalysis occurs in the initial transcripts of rRNA. The introns are capable of excising themselves by the process of two nucleophilic transesterification reactions. The RNA able to do this is sometimes referred to as a ribozyme. Additionally, the citric acid cycle is an autocatalytic cycle run in reverse. | ||
| + | |||
| + | 自我催化发生在 rna 的最初转录本中。这些内含子能够通过两个亲核酯交换反应反应自我激活。能够做到这一点的 RNA 有时被称为核酶。此外,三羧酸循环是一种反向运行的自动催化循环。 | ||
| + | |||
| + | :<math>\text{polymerization rate} \to \text{molecular weight}/\text{refractive index} \to \text{intensity}</math> | ||
| + | |||
| + | |||
| + | |||
| + | Ultimately, biological metabolism itself can be seen as a vast autocatalytic set, in that all of the molecular constituents of a biological cell are produced by reactions involving this same set of molecules. | ||
| + | |||
| + | 最终,生物的新陈代谢本身可以被看作是一个巨大的自我催化装置,因为一个生物细胞的所有分子成分都是由包含同一组分子的反应产生的。 | ||
| + | |||
| + | Noting that photo-polymerization rate is proportional to intensity<ref>{{Cite journal|last=Decker|first=Christian|date=1998-02-01|title=The use of UV irradiation in polymerization|url=|journal=Polymer International|language=en|volume=45|issue=2|pages=133–141 |doi=10.1002/(SICI)1097-0126(199802)45:2<133::AID-PI969>3.0.CO;2-F}}</ref> and that refractive index is proportional to molecular weight,<ref>{{Cite journal|last=Askadskii|first=A. A.|year=1990|title=Influence of crosslinking density on the properties of polymer networks|journal=Polymer Science U.S.S.R.|volume=32|issue=10|pages=2061–2069|doi=10.1016/0032-3950(90)90361-9}}</ref> the positive feedback between intensity and photo-polymerization establishes the auto-catalytic behavior. Optical auto-catalysis has been shown to result on spontaneous [[pattern formation]] in photopolymers.<ref>{{Cite journal|last=Burgess|first=Ian B.|last2=Shimmell|first2=Whitney E.|last3=Saravanamuttu|first3=Kalaichelvi|date=2007-04-01|title=Spontaneous Pattern Formation Due to Modulation Instability of Incoherent White Light in a Photopolymerizable Medium|journal=Journal of the American Chemical Society|volume=129|issue=15|pages=4738–4746|doi=10.1021/ja068967b|pmid=17378567|issn=0002-7863}}</ref><ref>{{Cite journal|last=Basker|first=Dinesh K.|last2=Brook|first2=Michael A.|last3=Saravanamuttu|first3=Kalaichelvi|title=Spontaneous Emergence of Nonlinear Light Waves and Self-Inscribed Waveguide Microstructure during the Cationic Polymerization of Epoxides|journal=The Journal of Physical Chemistry C|language=en|volume=119|issue=35|pages=20606–20617|doi=10.1021/acs.jpcc.5b07117|year=2015}}</ref><ref>{{Cite journal|last=Biria|first=Saeid|last2=Malley|first2=Philip P. A.|last3=Kahan|first3=Tara F.|last4=Hosein|first4=Ian D.|date=2016-03-03|title=Tunable Nonlinear Optical Pattern Formation and Microstructure in Cross-Linking Acrylate Systems during Free-Radical Polymerization|journal=The Journal of Physical Chemistry C|volume=120|issue=8|pages=4517–4528|doi=10.1021/acs.jpcc.5b11377|issn=1932-7447}}</ref> Hosein and co-workers discovered that optical autocatalysis can also occur in photoreactive polymer blends, and that the process can induce binary phase morphologies with the same pattern as the light profile.<ref name=":0" /> The light undergoes optical [[Modulational instability|modulation instability]], spontaneous dividing into a multitude of optical filaments, and the polymer system thereby forms filaments within the blend structure.<ref name=":0" /> The result is a new system that couples optical autocatalytic behavior to [[spinodal decomposition]]. | ||
| + | |||
| + | |||
| + | |||
| + | ==Biological example== | ||
| + | |||
| + | |||
| + | |||
| + | It is known that an important metabolic cycle, [[glycolysis]], displays temporal order.<ref>{{cite book | author=G. Nicolis and [[Ilya Prigogine]] | title=Self-Organization in Nonequilibrium Systems | location= New York | publisher=John Wiley and Sons| year=1977 | isbn=978-0-471-02401-9}} | ||
| + | |||
| + | </ref> Glycolysis consists of the degradation of one molecule of glucose and the overall production of two molecules of [[Adenosine triphosphate|ATP]]. The process is therefore of great importance to the energetics of living cells. The global glycolysis reaction involves [[glucose]], [[Adenosine diphosphate|ADP]], [[Nicotinamide adenine dinucleotide|NAD]], [[Pyruvic acid|pyruvate]], [[Adenosine triphosphate|ATP]], and NADH. | ||
| + | |||
| + | |||
| + | |||
| + | :<chem>glucose{} + 2ADP{} + 2P_\mathit{i}{} + 2NAD -> 2(pyruvate){} + 2ATP{} + 2NADH</chem>. | ||
| + | |||
| + | |||
| + | |||
| + | The details of the process are quite involved, however, a section of the process is autocatalyzed by [[phosphofructokinase]] (PFK). This portion of the process is responsible for oscillations in the pathway that lead to the process oscillating between an active and an inactive form. Thus, the autocatalytic reaction can modulate the process. | ||
| + | |||
| + | |||
| + | |||
| + | ==Shape tailoring of thin layers== | ||
| + | |||
| + | |||
| + | |||
| + | It is possible to use the results from an autocatalytic reaction coupled with [[reaction–diffusion system]] theory to tailor the design of a thin layer. The autocatalytic process allows controlling the nonlinear behavior of the oxidation [[Front (physics)|front]], which is used to establish the initial geometry needed to generate the arbitrary final geometry.<ref>{{cite journal |last1=Alfaro-Bittner |first1=K. |last2=Rojas |first2=R.G. |last3=Lafleur |first3=G. |last4=Calvez |first4=S. |last5=Almuneau |first5=G. |last6=Clerc |first6=M.G. |last7=Barbay |first7=S. |title=Modeling the Lateral Wet Oxidation of into Arbitrary Mesa Geometries |journal=Physical Review Applied|date=22 April 2019|volume=11 |issue=4|page=044067|doi=10.1103/PhysRevApplied.11.044067|url=https://journals.aps.org/prapplied/abstract/10.1103/PhysRevApplied.11.044067}}</ref> It has been successfully done in the wet oxidation of <math>Al_xGa_{1-x}As</math> to obtain arbitrary shaped layers of <math>AlO_x</math>. | ||
| + | |||
| + | |||
| + | |||
| + | ==Phase transitions== | ||
| + | |||
| + | |||
| + | |||
| + | The initial amounts of reactants determine the distance from a chemical equilibrium of the system. The greater the initial concentrations the further the system is from equilibrium. As the initial concentration increases, an abrupt change in [[entropy|order]] occurs. This abrupt change is known as [[phase transition]]. At the phase transition, fluctuations in macroscopic quantities, such as chemical concentrations, increase as the system oscillates between the more ordered state (lower entropy, such as ice) and the more disordered state (higher entropy, such as liquid water). Also, at the phase transition, macroscopic equations, such as the rate equations, fail. Rate equations can be derived from microscopic considerations. The derivations typically rely on a [[mean field theory]] approximation to microscopic dynamical equations. Mean field theory breaks down in the presence of large fluctuations (see [[Mean field theory]] article for a discussion). Therefore, since large fluctuations occur in the neighborhood of a phase transition, macroscopic equations, such as rate equations, fail. As the initial concentration increases further, the system settles into an ordered state in which fluctuations are again small. (see Prigogine reference) | ||
| + | |||
| + | |||
| + | |||
| + | ==Asymmetric autocatalysis== | ||
| + | |||
| + | |||
| + | |||
| + | Asymmetric autocatalysis occurs when the reaction product is [[chiral]] and thus acts as a chiral catalyst for its own production. Reactions of this type, such as the [[Soai reaction]], have the property that they can amplify a very small [[enantiomeric excess]] into a large one. This has been proposed as an important step in the origin of biological [[homochirality]].<ref name="Soai2001">{{cite journal|vauthors=Soai K, Sato I, Shibata T | title=Asymmetric autocatalysis and the origin of chiral homogeneity in organic compounds. | journal=The Chemical Record | year= 2001 | volume= 1 | issue= 4 | pages= 321–32 | pmid=11893072 | doi= 10.1002/tcr.1017| pmc= }}</ref> | ||
| + | |||
| + | |||
| + | |||
| + | == Role in origin of life == | ||
| + | |||
| + | |||
| + | |||
| + | {{main|Abiogenesis}} | ||
| + | |||
| + | In 1995 [[Stuart Kauffman]] proposed that life initially arose as autocatalytic chemical networks.<ref>{{cite book|author=Stuart Kauffman|title=At Home in the Universe: The Search for the Laws of Self-Organization and Complexity|isbn=978-0-19-509599-9|publisher=Oxford University Press|year=1995|url-access=registration|url=https://archive.org/details/athomeinuniverse00kauf_0}}</ref> | ||
| + | |||
| + | |||
Category:Catalysis | Category:Catalysis | ||
2020年10月26日 (一) 21:44的版本
此词条暂由彩云小译翻译,翻译字数共2466,未经人工整理和审校,带来阅读不便,请见谅。
A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction.[1] Such a reaction is called an autocatalytic reaction.
A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction. Such a reaction is called an autocatalytic reaction.
一个单一的化学反应,如果其中一个反应产物也是同一反应或耦合反应的催化剂,则称为自催化反应。这种反应称为自催化反应。
A set of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self-sustaining given an input of energy and food molecules (see autocatalytic set).
A set of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self-sustaining given an input of energy and food molecules (see autocatalytic set).
如果一系列化学反应作为反应产物产生足够多的其他反应的催化剂,使整套化学反应在能量和食物分子输入的情况下能够自我维持,则可以说这些化学反应是”集体自我催化”的。
Chemical reactions
A chemical reaction of two reactants and two products can be written as
A chemical reaction of two reactants and two products can be written as
两种反应物和两种产物的化学反应可以写成
- [math]\displaystyle{ \alpha A + \beta B \rightleftharpoons \sigma S + \tau T }[/math]
[math]\displaystyle{ \alpha A + \beta B \rightleftharpoons \sigma S + \tau T }[/math]
阿尔法 a + 贝塔 b 右旋/西格玛 s + tau t
where the Greek letters are stoichiometric coefficients and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily generalized to any number of reactants, products, and reactions.
where the Greek letters are stoichiometric coefficients and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily generalized to any number of reactants, products, and reactions.
其中希腊字母是化学计量系数,大写拉丁字母代表化学物种。化学反应在正向和反向两个方向进行。这个方程可以很容易地推广到任意数量的反应物、产物和反应。
Chemical equilibrium
In chemical equilibrium the forward and reverse reaction rates are such that each chemical species is being created at the same rate it is being destroyed. In other words, the rate of the forward reaction is equal to the rate of the reverse reaction.
In chemical equilibrium the forward and reverse reaction rates are such that each chemical species is being created at the same rate it is being destroyed. In other words, the rate of the forward reaction is equal to the rate of the reverse reaction.
在21世纪化学平衡,正向和反向反应的速率是如此之快,以至于每一种化学物质都以同样的速率被创造出来,同样的速率被摧毁。换句话说,前向反应的速率等于反向反应的速率。
- [math]\displaystyle{ k_+ [ A ]^\alpha [B ]^\beta = k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
[math]\displaystyle{ k_+ [ A ]^\alpha [B ]^\beta = k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
K _ + [ a ] ^ alpha [ b ] ^ beta = k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math >
Here, the brackets indicate the concentration of the chemical species, in moles per liter, and k+ and k− are rate constants.
Here, the brackets indicate the concentration of the chemical species, in moles per liter, and k+ and k− are rate constants.
这里,括号表示化学物质的浓度,以摩尔/升为单位,k < sub > + 和 k < sub >- 是速率常数。
Far from equilibrium
Far from equilibrium, the forward and reverse reaction rates no longer balance and the concentration of reactants and products is no longer constant. For every forward reaction [math]\displaystyle{ \alpha }[/math] molecules of A are destroyed. For every reverse reaction [math]\displaystyle{ \alpha }[/math] molecules of A are created. In the case of an elementary reaction step the reaction order in each direction equals the molecularity, so that the rate of change in the number of moles of A is then
Far from equilibrium, the forward and reverse reaction rates no longer balance and the concentration of reactants and products is no longer constant. For every forward reaction [math]\displaystyle{ \alpha }[/math] molecules of A are destroyed. For every reverse reaction [math]\displaystyle{ \alpha }[/math] molecules of A are created. In the case of an elementary reaction step the reaction order in each direction equals the molecularity, so that the rate of change in the number of moles of A is then
远离平衡,正向和反向反应速率不再平衡,反应物和产物的浓度不再是恒定的。对于每一个前向反应,a 的分子都会被破坏。对于每一个逆反应,a 的分子都会被创造出来。在基本反应步骤中,每个方向的反应级数等于分子量,因此 a 的摩尔数的变化率就是
- [math]\displaystyle{ {d \over dt}[ A ] =-\alpha k_+ [ A ]^\alpha [B ]^\beta +\alpha k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
[math]\displaystyle{ {d \over dt}[ A ] =-\alpha k_+ [ A ]^\alpha [B ]^\beta +\alpha k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
[ a ] =-alpha k _ + [ a ] ^ alpha [ b ] ^ beta + alpha k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math >
- [math]\displaystyle{ {d \over dt}[ B ] =-\beta k_+ [ A ]^\alpha [B ]^\beta +\beta k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
[math]\displaystyle{ {d \over dt}[ B ] =-\beta k_+ [ A ]^\alpha [B ]^\beta +\beta k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
[ b ] =-beta k _ + [ a ] ^ alpha [ b ] ^ beta + beta k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math >
- [math]\displaystyle{ {d \over dt}[ S ] =\sigma k_+ [ A ]^\alpha [B ]^\beta -\sigma k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
[math]\displaystyle{ {d \over dt}[ S ] =\sigma k_+ [ A ]^\alpha [B ]^\beta -\sigma k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
[ s ] = sigma k _ + [ a ] ^ alpha [ b ] ^ beta-sigma k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math >
- [math]\displaystyle{ {d \over dt}[ T ] =\tau k_+ [ A ]^\alpha [B ]^\beta -\tau k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
[math]\displaystyle{ {d \over dt}[ T ] =\tau k_+ [ A ]^\alpha [B ]^\beta -\tau k_{-} [S ]^\sigma[T ]^\tau \, }[/math]
[ t ] = tau k _ + [ a ] ^ alpha [ b ] ^ beta-tau k _ {-}[ s ] ^ sigma [ t ] ^ tau,</math >
This system of equations has a single stable fixed point when the forward rates and the reverse rates are equal (when [math]\displaystyle{ {d \over dt}=0 }[/math] for every species). This means that the system evolves to the equilibrium state, and this is the only state to which it evolves.[2]
This system of equations has a single stable fixed point when the forward rates and the reverse rates are equal (when [math]\displaystyle{ {d \over dt}=0 }[/math] for every species). This means that the system evolves to the equilibrium state, and this is the only state to which it evolves.
当正向速率和反向速率相等时,这个方程组有一个单一的稳定不动点(当每个物种的正向速率和反向速率相等时)。这意味着系统进化到平衡状态,而这是系统进化到的唯一状态。
Autocatalytic reactions
Sigmoid variation of product concentration in autocatalytic reactions
自催化反应中产物浓度的 s 形变化
Autocatalytic reactions are those in which at least one of the products is a reactant. Perhaps the simplest autocatalytic reaction can be written[1]
Autocatalytic reactions are those in which at least one of the products is a reactant. Perhaps the simplest autocatalytic reaction can be written
自催化反应是那些其中至少一个产物是反应物的反应。也许最简单的自催化反应可以写出来
- [math]\displaystyle{ A + B \rightleftharpoons 2B }[/math]
[math]\displaystyle{ [A]=\frac{[A]_0+[B]_0}{1+\frac{[B]_0}{[A]_0}e^{([A]_0+[B]_0)kt}} }[/math]
< math > [ a ] = frac {[ a ] _ 0 + [ b ] _ 0}{1 + frac {[ b ] _ 0}{[ a ] _ 0} e ^ {(([ a ] _ 0 + [ b ] _ 0) kt }} </math >
and
及
with the rate equations (for an elementary reaction)
[math]\displaystyle{ [B]=\frac{[A]_0+[B]_0}{1+\frac{[A]_0}{[B]_0}e^{-([A]_0+[B]_0)kt}} }[/math].
< math > [ b ] = frac {[ a ] _ 0 + [ b ] _ 0}{1 + frac {[ a ] _ 0}{[ b ] _ 0} e ^ {-([ a ] _ 0 + [ b ] _ 0) kt }} </math > .
- [math]\displaystyle{ {d \over dt}[ A ] =- k_+ [ A ] [B ] + k_{-} [B ]^2 \, }[/math]
The graph for these equations is a sigmoid curve (specifically a logistic function), which is typical for autocatalytic reactions: these chemical reactions proceed slowly at the start (the induction period) because there is little catalyst present, the rate of reaction increases progressively as the reaction proceeds as the amount of catalyst increases and then it again slows down as the reactant concentration decreases. If the concentration of a reactant or product in an experiment follows a sigmoid curve, the reaction may be autocatalytic.
这些方程的图表是一个 s 形曲线(特别是一个 Logistic函数) ,这是典型的自催化反应: 这些化学反应进行缓慢的开始(诱导期) ,因为有少量的催化剂存在,反应速度逐步增加,随着反应进行的催化剂数量增加,然后它再次减缓,作为反应物浓度降低。如果实验中反应物或产物的浓度符合 s 形曲线,则反应可能是自催化的。
- [math]\displaystyle{ {d \over dt}[ B ] = + k_+ [ A ] [B ] -k_{-} [B ]^2 \, }[/math].
These kinetic equations apply for example to the acid-catalyzed hydrolysis of some esters to carboxylic acids and alcohols. In the hurricane example, hurricanes are formed from unequal heating within the atmosphere. The Earth's atmosphere is then far from thermal equilibrium. The order of the Earth's atmosphere increases, but at the expense of the order of the sun. The sun is becoming more disorderly as it ages and throws off light and material to the rest of the universe. The total disorder of the sun and the earth increases despite the fact that orderly hurricanes are generated on earth.
这些动力学方程适用于某些酯类在酸催化下水解为羧酸和醇类。在飓风的例子中,飓风是由大气中不均匀的热量形成的。地球的大气层远离热平衡。地球大气层的次序增加了,但代价是太阳的次序。随着年龄的增长,太阳将光线和物质抛向宇宙的其他部分,它正变得越来越无序。尽管有序的飓风在地球上产生,但太阳和地球的总体混乱程度仍在增加。
This reaction is one in which a molecule of species A interacts with a molecule of species B. The A molecule is converted into a B molecule. The final product consists of the original B molecule plus the B molecule created in the reaction.
A similar example exists for living chemical systems. The sun provides energy to green plants. The green plants are food for other living chemical systems. The energy absorbed by plants and converted into chemical energy generates a system on earth that is orderly and far from chemical equilibrium. Here, the difference from chemical equilibrium is determined by an excess of reactants over the equilibrium amount. Once again, order on earth is generated at the expense of entropy increase of the sun. The total entropy of the earth and the rest of the universe increases, consistent with the Second Law.
类似的例子也存在于活的化学系统中。太阳为绿色植物提供能量。绿色植物是其他生命化学系统的食物。植物吸收并转化为化学能的能量在地球上形成了一个有序的系统,这个系统远离化学平衡。在这里,反应物超过平衡量决定了化学平衡的差异。再一次,地球上的秩序是以太阳的熵增为代价而产生的。地球和宇宙其余部分的总熵增加,符合第二定律。
The key feature of these rate equations is that they are nonlinear; the second term on the right varies as the square of the concentration of B. This feature can lead to multiple fixed points of the system, much like a quadratic equation can have multiple roots. Multiple fixed points allow for multiple states of the system. A system existing in multiple macroscopic states is more orderly (has lower entropy) than a system in a single state.
Some autocatalytic reactions also generate order in a system at the expense of its surroundings. For example, (clock reactions) have intermediates whose concentrations oscillate in time, corresponding to temporal order. Other reactions generate spatial separation of chemical species corresponding to spatial order. More complex reactions are involved in metabolic pathways and metabolic networks in biological systems.
一些自催化反应也以牺牲周围环境为代价,在系统中产生有序。例如,(时钟反应)有其浓度在时间上振荡的中间体,对应于时间顺序。其他反应产生空间分离的化学物质对应的空间秩序。更复杂的反应涉及到生物系统中的代谢途径和代谢网络。
The concentrations of A and B vary in time according to[1][3]
- [math]\displaystyle{ [A]=\frac{[A]_0+[B]_0}{1+\frac{[B]_0}{[A]_0}e^{([A]_0+[B]_0)kt}} }[/math]
The transition to order as the distance from equilibrium increases is not usually continuous. Order typically appears abruptly. The threshold between the disorder of chemical equilibrium and order is known as a phase transition. The conditions for a phase transition can be determined with the mathematical machinery of non-equilibrium thermodynamics.
随着离平衡距离的增加,向有序的过渡通常是不连续的。秩序通常会突然出现。化学平衡和有序之间的无序阈值被称为相变。相变的条件可以用非平衡态热力学的数学方法来确定。
and
- [math]\displaystyle{ [B]=\frac{[A]_0+[B]_0}{1+\frac{[A]_0}{[B]_0}e^{-([A]_0+[B]_0)kt}} }[/math].
The graph for these equations is a sigmoid curve (specifically a logistic function), which is typical for autocatalytic reactions: these chemical reactions proceed slowly at the start (the induction period) because there is little catalyst present, the rate of reaction increases progressively as the reaction proceeds as the amount of catalyst increases and then it again slows down as the reactant concentration decreases. If the concentration of a reactant or product in an experiment follows a sigmoid curve, the reaction may be autocatalytic.
A chemical reaction cannot oscillate about a position of final equilibrium because the second law of thermodynamics requires that a thermodynamic system approach equilibrium and not recede from it. For a closed system at constant temperature and pressure, the Gibbs free energy must decrease continuously and not oscillate. However it is possible that the concentrations of some reaction intermediates oscillate, and also that the rate of formation of products oscillates.
一个化学反应不能在一个最终平衡位置上振荡,因为热力学第二定律需要一个接近平衡的热力学系统,而不能退出平衡位置。对于一个温度和压力恒定的封闭系统,吉布斯自由能必须不断减小而不振荡。然而,一些反应中间体的浓度可能会振荡,产物的生成速率也可能振荡。
These kinetic equations apply for example to the acid-catalyzed hydrolysis of some esters to carboxylic acids and alcohols.[3] There must be at least some acid present initially to start the catalyzed mechanism; if not the reaction must start by an alternate uncatalyzed path which is usually slower. The above equations for the catalyzed mechanism would imply that the concentration of acid product remains zero forever.[3]
The Lotka–Volterra equation is isomorphic with the predator–prey model and the two-reaction autocatalytic model. In this example baboons and cheetahs are equivalent to two different chemical species X and Y in autocatalytic reactions.
方程[同构于捕食-食饵模型和双反应自催化模型。在这个例子中,狒狒和猎豹在自动催化反应中相当于两种不同的化学物种 x 和 y
Creation of order
Consider a coupled set of two autocatalytic reactions in which the concentration of one of the reactants A is much larger than its equilibrium value. In this case, the forward reaction rate is so much larger than the reverse rates that we can neglect the reverse rates.
考虑一组耦合的两个自催化反应,其中一个反应物 a 的浓度远远大于其平衡值。在这种情况下,正向反应速率远远大于反向反应速率,因此我们可以忽略反向反应速率。
Background
[math]\displaystyle{ A + X \rightarrow 2X }[/math]
A + x 右行2X
The second law of thermodynamics states that the disorder (entropy) of a physical or chemical system and its surroundings (a closed system) must increase with time. Systems left to themselves become increasingly random, and orderly energy of a system like uniform motion degrades eventually to the random motion of particles in a heat bath.
[math]\displaystyle{ X + Y \rightarrow 2Y }[/math]
(数学) x + y 右行2Y
There are, however, many instances in which physical systems spontaneously become emergent or orderly. For example, despite the destruction they cause, hurricanes have a very orderly vortex motion when compared to the random motion of the air molecules in a closed room. Even more spectacular is the order created by chemical systems; the most dramatic being the order associated with life.
[math]\displaystyle{ Y \rightarrow E }[/math]
[数学,数学]
This is consistent with the Second Law, which requires that the total disorder of a system and its surroundings must increase with time. Order can be created in a system by an even greater decrease in order of the system's surroundings.[4] In the hurricane example, hurricanes are formed from unequal heating within the atmosphere. The Earth's atmosphere is then far from thermal equilibrium. The order of the Earth's atmosphere increases, but at the expense of the order of the sun. The sun is becoming more disorderly as it ages and throws off light and material to the rest of the universe. The total disorder of the sun and the earth increases despite the fact that orderly hurricanes are generated on earth.
[math]\displaystyle{ {d \over dt}[ X ] = k_1 [ A ] [X ] - k_{2} [X ][Y ] \, }[/math]
< math > { d over dt }[ x ] = k_1[ a ][ x ]-k_2}[ x ][ y ] ,</math >
A similar example exists for living chemical systems. The sun provides energy to green plants. The green plants are food for other living chemical systems. The energy absorbed by plants and converted into chemical energy generates a system on earth that is orderly and far from chemical equilibrium. Here, the difference from chemical equilibrium is determined by an excess of reactants over the equilibrium amount. Once again, order on earth is generated at the expense of entropy increase of the sun. The total entropy of the earth and the rest of the universe increases, consistent with the Second Law.
[math]\displaystyle{ {d \over dt}[ Y ] = k_2 [ X ] [Y ] - k_{3} [Y ] \, }[/math].
[ y ] = k _ 2[ x ][ y ]-k _ {3}[ y ] ,[/math ].
Some autocatalytic reactions also generate order in a system at the expense of its surroundings. For example, (clock reactions) have intermediates whose concentrations oscillate in time, corresponding to temporal order. Other reactions generate spatial separation of chemical species corresponding to spatial order. More complex reactions are involved in metabolic pathways and metabolic networks in biological systems.
Here, we have neglected the depletion of the reactant A, since its concentration is so large. The rate constants for the three reactions are [math]\displaystyle{ k_1 }[/math], [math]\displaystyle{ k_2 }[/math], and [math]\displaystyle{ k_3 }[/math], respectively.
在这里,我们忽略了反应物 a 的耗尽,因为它的浓度很大。这三个反应的速率常数分别是 < math > k _ 1 </math > ,< math > k _ 2 </math > ,和 < math > k _ 3 </math > 。
The transition to order as the distance from equilibrium increases is not usually continuous. Order typically appears abruptly. The threshold between the disorder of chemical equilibrium and order is known as a phase transition. The conditions for a phase transition can be determined with the mathematical machinery of non-equilibrium thermodynamics.
This system of rate equations is known as the Lotka–Volterra equation and is most closely associated with population dynamics in predator–prey relationships. This system of equations can yield oscillating concentrations of the reaction intermediates X and Y. The amplitude of the oscillations depends on the concentration of A (which decreases without oscillation). Such oscillations are a form of emergent temporal order that is not present in equilibrium.
这个速率方程组被称为 Lotka-Volterra 方程,在捕食者-食饵关系中与族群动态最密切相关。这个方程组可以产生反应中间体 x 和 y 的振荡浓度。振荡的振幅取决于 a 的浓度(a 的浓度下降而没有振荡)。这种振荡是一种涌现的时间顺序,在平衡中不存在。
Temporal order
A chemical reaction cannot oscillate about a position of final equilibrium because the second law of thermodynamics requires that a thermodynamic system approach equilibrium and not recede from it. For a closed system at constant temperature and pressure, the Gibbs free energy must decrease continuously and not oscillate. However it is possible that the concentrations of some reaction intermediates oscillate, and also that the rate of formation of products oscillates.[5]
Idealized example: Lotka–Volterra equation
Another example of a system that demonstrates temporal order is the Brusselator (see Prigogine reference). It is characterized by the reactions
另一个演示时间顺序的系统例子是 Brusselator (见 Prigogine 参考文献)。人们的反应是拥有属性的
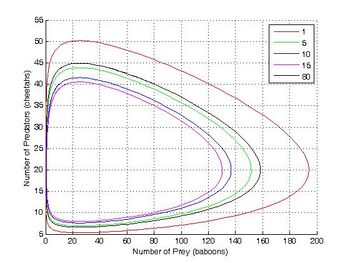
Consider a coupled set of two autocatalytic reactions in which the concentration of one of the reactants A is much larger than its equilibrium value. In this case, the forward reaction rate is so much larger than the reverse rates that we can neglect the reverse rates.
[math]\displaystyle{ A \rightarrow X }[/math]
《数学》 a right tarrow x
- [math]\displaystyle{ A + X \rightarrow 2X }[/math]
[math]\displaystyle{ 2X + Y \rightarrow 3X }[/math]
2X + y 右行3X
- [math]\displaystyle{ X + Y \rightarrow 2Y }[/math]
[math]\displaystyle{ B + X \rightarrow Y + D }[/math]
B + x 右边 y + d
- [math]\displaystyle{ Y \rightarrow E }[/math]
[math]\displaystyle{ X \rightarrow E }[/math]
X 右边的 e
with the rate equations
with the rate equations
用速率方程式
- [math]\displaystyle{ {d \over dt}[ X ] = k_1 [ A ] [X ] - k_{2} [X ][Y ] \, }[/math]
[math]\displaystyle{ {d \over dt}[ X ] = [A ] + [ X ]^2 [Y ] - [B ] [X ] - [X ] \, }[/math]
< math > { d over dt }[ x ] = [ a ] + [ x ] ^ 2[ y ]-[ b ][ x ]-[ x ] ,</math >
- [math]\displaystyle{ {d \over dt}[ Y ] = k_2 [ X ] [Y ] - k_{3} [Y ] \, }[/math].
[math]\displaystyle{ {d \over dt}[ Y ] = [B ] [X ] - [ X ]^2 [Y ] \, }[/math]
[数学]{ d/dt }[ y ] = [ b ][ x ]-[ x ] ^ 2[ y ] ,[/math ]
Here, we have neglected the depletion of the reactant A, since its concentration is so large. The rate constants for the three reactions are [math]\displaystyle{ k_1 }[/math], [math]\displaystyle{ k_2 }[/math], and [math]\displaystyle{ k_3 }[/math], respectively.
where, for convenience, the rate constants have been set to 1.
为了方便起见,速率常数设置为1。
This system of rate equations is known as the Lotka–Volterra equation and is most closely associated with population dynamics in predator–prey relationships. This system of equations can yield oscillating concentrations of the reaction intermediates X and Y. The amplitude of the oscillations depends on the concentration of A (which decreases without oscillation). Such oscillations are a form of emergent temporal order that is not present in equilibrium.
The Brusselator in the unstable regime. A=1. B=2.5. X(0)=1. Y(0)=0. The system approaches a [[limit cycle. For B<1+A the system is stable and approaches a fixed point.]]
不稳定政权中的布鲁塞尔人。1.2.5.X (0) = 1.Y (0) = 0.该系统接近[极限环]。对于 b < 1 + a,系统是稳定的,并且接近一个固定点。]
The Brusselator has a fixed point at
布鲁塞尔终结者有一个固定点
Another idealized example: Brusselator
[math]\displaystyle{ [ X ] = A \, }[/math]
[数学] = a,[数学]
Another example of a system that demonstrates temporal order is the Brusselator (see Prigogine reference). It is characterized by the reactions
[math]\displaystyle{ [ Y ] = {B \over A} \, }[/math].
[ math ][ y ] = { b over a } ,[ math ].
- [math]\displaystyle{ A \rightarrow X }[/math]
The fixed point becomes unstable when
定点变得不稳定,当
- [math]\displaystyle{ 2X + Y \rightarrow 3X }[/math]
[math]\displaystyle{ B\gt 1+A^2 \, }[/math]
1 + a ^ 2,
- [math]\displaystyle{ B + X \rightarrow Y + D }[/math]
leading to an oscillation of the system. Unlike the Lotka-Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a limit cycle.
导致了系统的振荡。与洛特卡-沃尔泰拉方程不同,布鲁塞尔振荡器的振荡并不取决于最初反应物的数量。相反,在足够的时间之后,振荡接近极限环。
- [math]\displaystyle{ X \rightarrow E }[/math]
with the rate equations
An idealized example of spatial spontaneous symmetry breaking is the case in which we have two boxes of material separated by a permeable membrane so that material can diffuse between the two boxes. It is assumed that identical Brusselators are in each box with nearly identical initial conditions. (see Prigogine reference)
一个理想化的空间自发对称性破缺的例子是,我们有两个盒子的材料被一个可渗透的薄膜分开,这样材料可以在两个盒子之间扩散。我们假设在每个盒子中都有相同的布鲁塞尔子,并且具有几乎相同的初始条件。(见 Prigogine 参考文献)
- [math]\displaystyle{ {d \over dt}[ X ] = [A ] + [ X ]^2 [Y ] - [B ] [X ] - [X ] \, }[/math]
[math]\displaystyle{ {d \over dt}[ X_1 ] = [A ] + [ X _1]^2 [Y_1 ] - [B ] [X_1 ] - [X_1 ] + D_x\left( X_2 - X_1 \right)\, }[/math]
< math > { d over dt }[ x _ 1] = [ a ] + [ x _ 1] ^ 2[ y _ 1]-[ b ][ x _ 1]-[ x _ 1] + d _ x 左(x _ 2-x _ 1右) ,</math >
- [math]\displaystyle{ {d \over dt}[ Y ] = [B ] [X ] - [ X ]^2 [Y ] \, }[/math]
[math]\displaystyle{ {d \over dt}[ Y_1 ] = [B ] [X_1 ] - [ X_1 ]^2 [Y_1 ] + D_y\left( Y_2 - Y_1\right) \, }[/math]
< math > { d over dt }[ y _ 1] = [ b ][ x _ 1]-[ x _ 1] ^ 2[ y _ 1] + d _ y 左(y _ 2-y _ 1右) ,</math >
where, for convenience, the rate constants have been set to 1.
[math]\displaystyle{ {d \over dt}[ X_2 ] = [A ] + [ X _2]^2 [Y_2 ] - [B ] [X_2 ] - [X_2 ] + D_x\left( X_1 - X_2 \right)\, }[/math]
[ x _ 2] = [ a ] + [ x _ 2] ^ 2[ y _ 2]-[ b ][ x _ 2]-[ x _ 2] + d _ x 左(x _ 1-x _ 2右) ,</math >
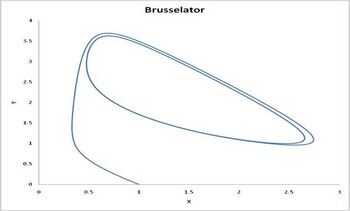
[math]\displaystyle{ {d \over dt}[ Y_2 ] = [B ] [X_2 ] - [ X_2 ]^2 [Y_2 ] + D_y\left( Y_1 - Y_2\right) \, }[/math]
[ math ]{ d over dt }[ y _ 2] = [ b ][ x _ 2]-[ x _ 2] ^ 2[ y _ 2] + d _ y 左(y _ 1-y _ 2右) ,</math >
The Brusselator has a fixed point at
Here, the numerical subscripts indicate which box the material is in. There are additional terms proportional to the diffusion coefficient D that account for the exchange of material between boxes.
在这里,数字下标表示材料在哪个盒子里。还有一些与扩散系数 d 成比例的附加项,用于解释盒子之间的物质交换。
- [math]\displaystyle{ [ X ] = A \, }[/math]
If the system is initiated with the same conditions in each box, then a small fluctuation will lead to separation of materials between the two boxes. One box will have a predominance of X, and the other will have a predominance of Y.
如果系统启动时每个箱子的条件相同,那么一个小的波动将导致两个箱子之间的物料分离。一个盒子将具有 x 的优势,而另一个盒子将具有 y 的优势。
- [math]\displaystyle{ [ Y ] = {B \over A} \, }[/math].
The fixed point becomes unstable when
Real examples of clock reactions are the Belousov–Zhabotinsky reaction (BZ reaction), the Briggs–Rauscher reaction, the Bray–Liebhafsky reaction and the iodine clock reaction. These are oscillatory reactions, and the concentration of products and reactants can be approximated in terms of damped oscillations.
时钟反应的真实例子是 Belousov-Zhabotinsky 反应(BZ 反应) ,Briggs-Rauscher 反应,Bray-Liebhafsky 反应和碘钟反应。这些都是振荡反应,产物和反应物的浓度可以用阻尼振荡来近似。
- [math]\displaystyle{ B\gt 1+A^2 \, }[/math]
The best-known reaction, the BZ reaction, can be created with a mixture of potassium bromate [math]\ce{ (KBrO3) }[/math], malonic acid [math]\ce{ (CH2(COOH)2) }[/math], and manganese sulfate [math]\ce{ (MnSO4) }[/math] prepared in a heated solution with sulfuric acid [math]\ce{ (H2SO4) }[/math] as solvent.
其中最著名的反应是 BZ 反应,它是在硫酸 </chem > (H2SO4) </chem > (H2SO4) </chem > (CH2(COOH)2) </chem > 和用硫酸 </chem > (MnSO4) </chem > 加热溶液中制得的混合溴酸钾。
leading to an oscillation of the system. Unlike the Lotka-Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a limit cycle.[6]
[math]\ce{ glucose{} + 2ADP{} + 2P_\mathit{i}{} + 2NAD -> 2(pyruvate){} + 2ATP{} + 2NADH }[/math].
< chem > glucose {} + 2ADP {} + 2P _ mathit { i }{} + 2NAD-> 2(丙酮酸盐){} + 2ATP {} + 2NADH </chem > 。
Spatial order
The details of the process are quite involved, however, a section of the process is autocatalyzed by phosphofructokinase (PFK). This portion of the process is responsible for oscillations in the pathway that lead to the process oscillating between an active and an inactive form. Thus, the autocatalytic reaction can modulate the process.
该过程的细节相当复杂,然而,该过程的一部分是由磷酸果糖激酶(PFK)自催化的。这部分的过程负责振荡的路径,导致过程之间的振荡活跃和非活跃的形式。因此,自催化反应可以调节这一过程。
An idealized example of spatial spontaneous symmetry breaking is the case in which we have two boxes of material separated by a permeable membrane so that material can diffuse between the two boxes. It is assumed that identical Brusselators are in each box with nearly identical initial conditions. (see Prigogine reference)
- [math]\displaystyle{ {d \over dt}[ X_1 ] = [A ] + [ X _1]^2 [Y_1 ] - [B ] [X_1 ] - [X_1 ] + D_x\left( X_2 - X_1 \right)\, }[/math]
It is possible to use the results from an autocatalytic reaction coupled with reaction–diffusion system theory to tailor the design of a thin layer. The autocatalytic process allows controlling the nonlinear behavior of the oxidation front, which is used to establish the initial geometry needed to generate the arbitrary final geometry. It has been successfully done in the wet oxidation of [math]\displaystyle{ Al_xGa_{1-x}As }[/math] to obtain arbitrary shaped layers of [math]\displaystyle{ AlO_x }[/math].
利用自催化反应结合反应扩散系统理论的结果来设计薄层是可能的。自催化过程允许控制氧化前沿的非线性行为,用于建立生成任意最终几何所需的初始几何。在湿式氧化法中,成功地获得了任意形状的氧化铝层。
- [math]\displaystyle{ {d \over dt}[ Y_1 ] = [B ] [X_1 ] - [ X_1 ]^2 [Y_1 ] + D_y\left( Y_2 - Y_1\right) \, }[/math]
- [math]\displaystyle{ {d \over dt}[ X_2 ] = [A ] + [ X _2]^2 [Y_2 ] - [B ] [X_2 ] - [X_2 ] + D_x\left( X_1 - X_2 \right)\, }[/math]
The initial amounts of reactants determine the distance from a chemical equilibrium of the system. The greater the initial concentrations the further the system is from equilibrium. As the initial concentration increases, an abrupt change in order occurs. This abrupt change is known as phase transition. At the phase transition, fluctuations in macroscopic quantities, such as chemical concentrations, increase as the system oscillates between the more ordered state (lower entropy, such as ice) and the more disordered state (higher entropy, such as liquid water). Also, at the phase transition, macroscopic equations, such as the rate equations, fail. Rate equations can be derived from microscopic considerations. The derivations typically rely on a mean field theory approximation to microscopic dynamical equations. Mean field theory breaks down in the presence of large fluctuations (see Mean field theory article for a discussion). Therefore, since large fluctuations occur in the neighborhood of a phase transition, macroscopic equations, such as rate equations, fail. As the initial concentration increases further, the system settles into an ordered state in which fluctuations are again small. (see Prigogine reference)
反应物的初始数量决定了反应体系到化学平衡的距离。初始浓度越大,系统离平衡越远。随着初始浓度的增加,有序发生突变。这种突然的变化称为相变。在相变过程中,宏观量(如化学浓度)的涨落会随着系统在更有序的状态(如冰)和更无序的状态(如液态水)之间振荡而增加。此外,在相变时,宏观方程,如速率方程,失败。速率方程可以从微观考虑推导出来。推导通常依赖于平均场理论对微观动力学方程的近似。在存在大的波动时,平均场理论崩溃了(见平均场理论文章的讨论)。因此,由于大的波动发生在附近的相变,宏观方程,如速率方程,失败。随着初始浓度的进一步增加,系统进入有序状态,波动再次变小。(见 Prigogine 参考文献)
- [math]\displaystyle{ {d \over dt}[ Y_2 ] = [B ] [X_2 ] - [ X_2 ]^2 [Y_2 ] + D_y\left( Y_1 - Y_2\right) \, }[/math]
Here, the numerical subscripts indicate which box the material is in. There are additional terms proportional to the diffusion coefficient D that account for the exchange of material between boxes.
Asymmetric autocatalysis occurs when the reaction product is chiral and thus acts as a chiral catalyst for its own production. Reactions of this type, such as the Soai reaction, have the property that they can amplify a very small enantiomeric excess into a large one. This has been proposed as an important step in the origin of biological homochirality.
不对称自催化反应发生在反应产物是手性的情况下,因此可以作为手性催化剂自身生产。这种类型的反应,比如 Soai 反应,具有将非常小的对映体过量百分数放大成大的反应的特性。这被认为是生物同手性起源的重要步骤。
If the system is initiated with the same conditions in each box, then a small fluctuation will lead to separation of materials between the two boxes. One box will have a predominance of X, and the other will have a predominance of Y.
Real examples
Real examples of clock reactions are the Belousov–Zhabotinsky reaction (BZ reaction), the Briggs–Rauscher reaction, the Bray–Liebhafsky reaction and the iodine clock reaction. These are oscillatory reactions, and the concentration of products and reactants can be approximated in terms of damped oscillations.
In 1995 Stuart Kauffman proposed that life initially arose as autocatalytic chemical networks.
1995年,斯图尔特 · 考夫曼提出生命最初是以自催化化学网络的形式出现的。
The best-known reaction, the BZ reaction, can be created with a mixture of potassium bromate [math]\ce{ (KBrO3) }[/math], malonic acid [math]\ce{ (CH2(COOH)2) }[/math], and manganese sulfate [math]\ce{ (MnSO4) }[/math] prepared in a heated solution with sulfuric acid [math]\ce{ (H2SO4) }[/math] as solvent.[7]
British ethologist Richard Dawkins wrote about autocatalysis as a potential explanation for abiogenesis in his 2004 book The Ancestor's Tale. He cites experiments performed by Julius Rebek and his colleagues at the Scripps Research Institute in California in which they combined amino adenosine and pentafluorophenyl ester with the autocatalyst amino adenosine triacid ester (AATE). One system from the experiment contained variants of AATE which catalyzed the synthesis of themselves. This experiment demonstrated the possibility that autocatalysts could exhibit competition within a population of entities with heredity, which could be interpreted as a rudimentary form of natural selection, and that certain environmental changes (such as irradiation) could alter the chemical structure of some of these self-replicating molecules (an analog for mutation) in such ways that could either boost or interfere with its ability to react, thus boosting or interfering with its ability to replicate and spread in the population.
英国动物行为学家理查德 · 道金斯在他2004年出版的《祖先的故事》一书中提到了自我催化作为自然发生的潜在解释。他引用了 Julius Rebek 和他的同事们在加利福尼亚斯克里普斯研究所进行的实验,他们将氨基腺苷和五氟苯酯与氨基腺苷三酸酯(AATE)结合在一起。实验中的一个系统包含了催化自身合成的 AATE 的变体。这项实验证明了这样一种可能性,即自动催化剂可以在具有遗传性的实体群体中展现竞争,这可以被解释为一种基本的自然选择形式,而且某些环境变化(如辐照)可以改变某些自我复制分子(变异的类似物)的化学结构,这种方式可以增强或干扰其反应能力,从而增强或干扰其复制和在群体中传播的能力。
Optics example
Autocatalysis plays a major role in the processes of life. Two researchers who have emphasized its role in the origins of life are Robert Ulanowicz and Stuart Kauffman.
自动催化在生命过程中起着重要的作用。强调罗伯特·尤兰维奇在生命起源中的作用的两位研究人员是斯图尔特 · 考夫曼和斯图尔特 · 考夫曼。
Another autocatalytic system is one driven by light coupled to photo-polymerization reactions. In a process termed optical autocatalysis, positive feedback is created between light intensity and photo-polymerization rate, via polymerization-induced increases in the refractive index. Light's preference to occupy regions of higher refractive index results in leakage of light into regions of higher molecular weight, thereby amplifying the photo-chemical reaction. The positive feedback may be expressed as:[8]
Autocatalysis occurs in the initial transcripts of rRNA. The introns are capable of excising themselves by the process of two nucleophilic transesterification reactions. The RNA able to do this is sometimes referred to as a ribozyme. Additionally, the citric acid cycle is an autocatalytic cycle run in reverse.
自我催化发生在 rna 的最初转录本中。这些内含子能够通过两个亲核酯交换反应反应自我激活。能够做到这一点的 RNA 有时被称为核酶。此外,三羧酸循环是一种反向运行的自动催化循环。
- [math]\displaystyle{ \text{polymerization rate} \to \text{molecular weight}/\text{refractive index} \to \text{intensity} }[/math]
Ultimately, biological metabolism itself can be seen as a vast autocatalytic set, in that all of the molecular constituents of a biological cell are produced by reactions involving this same set of molecules.
最终,生物的新陈代谢本身可以被看作是一个巨大的自我催化装置,因为一个生物细胞的所有分子成分都是由包含同一组分子的反应产生的。
Noting that photo-polymerization rate is proportional to intensity[9] and that refractive index is proportional to molecular weight,[10] the positive feedback between intensity and photo-polymerization establishes the auto-catalytic behavior. Optical auto-catalysis has been shown to result on spontaneous pattern formation in photopolymers.[11][12][13] Hosein and co-workers discovered that optical autocatalysis can also occur in photoreactive polymer blends, and that the process can induce binary phase morphologies with the same pattern as the light profile.[8] The light undergoes optical modulation instability, spontaneous dividing into a multitude of optical filaments, and the polymer system thereby forms filaments within the blend structure.[8] The result is a new system that couples optical autocatalytic behavior to spinodal decomposition.
Biological example
It is known that an important metabolic cycle, glycolysis, displays temporal order.[14] Glycolysis consists of the degradation of one molecule of glucose and the overall production of two molecules of ATP. The process is therefore of great importance to the energetics of living cells. The global glycolysis reaction involves glucose, ADP, NAD, pyruvate, ATP, and NADH.
- [math]\ce{ glucose{} + 2ADP{} + 2P_\mathit{i}{} + 2NAD -> 2(pyruvate){} + 2ATP{} + 2NADH }[/math].
The details of the process are quite involved, however, a section of the process is autocatalyzed by phosphofructokinase (PFK). This portion of the process is responsible for oscillations in the pathway that lead to the process oscillating between an active and an inactive form. Thus, the autocatalytic reaction can modulate the process.
Shape tailoring of thin layers
It is possible to use the results from an autocatalytic reaction coupled with reaction–diffusion system theory to tailor the design of a thin layer. The autocatalytic process allows controlling the nonlinear behavior of the oxidation front, which is used to establish the initial geometry needed to generate the arbitrary final geometry.[15] It has been successfully done in the wet oxidation of [math]\displaystyle{ Al_xGa_{1-x}As }[/math] to obtain arbitrary shaped layers of [math]\displaystyle{ AlO_x }[/math].
Phase transitions
The initial amounts of reactants determine the distance from a chemical equilibrium of the system. The greater the initial concentrations the further the system is from equilibrium. As the initial concentration increases, an abrupt change in order occurs. This abrupt change is known as phase transition. At the phase transition, fluctuations in macroscopic quantities, such as chemical concentrations, increase as the system oscillates between the more ordered state (lower entropy, such as ice) and the more disordered state (higher entropy, such as liquid water). Also, at the phase transition, macroscopic equations, such as the rate equations, fail. Rate equations can be derived from microscopic considerations. The derivations typically rely on a mean field theory approximation to microscopic dynamical equations. Mean field theory breaks down in the presence of large fluctuations (see Mean field theory article for a discussion). Therefore, since large fluctuations occur in the neighborhood of a phase transition, macroscopic equations, such as rate equations, fail. As the initial concentration increases further, the system settles into an ordered state in which fluctuations are again small. (see Prigogine reference)
Asymmetric autocatalysis
Asymmetric autocatalysis occurs when the reaction product is chiral and thus acts as a chiral catalyst for its own production. Reactions of this type, such as the Soai reaction, have the property that they can amplify a very small enantiomeric excess into a large one. This has been proposed as an important step in the origin of biological homochirality.[16]
Role in origin of life
In 1995 Stuart Kauffman proposed that life initially arose as autocatalytic chemical networks.[17]
Category:Catalysis
类别: 催化
This page was moved from wikipedia:en:Autocatalysis. Its edit history can be viewed at 自催化/edithistory
- ↑ 1.0 1.1 1.2 Steinfeld J.I., Francisco J.S. and Hase W.L. Chemical Kinetics and Dynamics (2nd ed., Prentice-Hall 1999) p.151-2
- ↑ Ross, John; Garcia-Colin, Leopoldo S. (March 1989). "Thermodynamics of chemical systems far from equilibrium". The Journal of Physical Chemistry. 93 (5): 2091–2092. doi:10.1021/j100342a075.
- ↑ 3.0 3.1 3.2 Moore J.W. and Pearson R.G. Kinetics and Mechanism (John Wiley 1981) p.26
- ↑ Ilya Prigogine (1980). From Being to Becoming: Time and Complexity in the Physical Sciences. San Francisco: W. H. Freeman. ISBN 978-0-7167-1107-0. https://archive.org/details/frombeingtobecom00ipri. with the rate equations 用速率方程式
- ↑ Espenson, J.H. Chemical Kinetics and Reaction Mechanisms (2nd ed., McGraw-Hill 2002) p.190
- ↑ "Archived copy Another autocatalytic system is one driven by light coupled to photo-polymerization reactions. In a process termed optical autocatalysis, positive feedback is created between light intensity and photo-polymerization rate, via polymerization-induced increases in the refractive index. Light's preference to occupy regions of higher refractive index results in leakage of light into regions of higher molecular weight, thereby amplifying the photo-chemical reaction. The positive feedback may be expressed as: 另一种自催化体系是由光与光聚合反应耦合驱动的体系。在一个被称为光学自催化的过程中,光强度和光聚合速率之间产生正反馈,通过聚合诱导增加折射率。光喜欢占据折射率较高的区域,导致光线泄漏到分子量较高的区域,从而放大了光化学反应。积极的反馈可以表示为:" (PDF). Archived from the original (PDF) on 2008-12-17
Noting that photo-polymerization rate is proportional to intensity and that refractive index is proportional to molecular weight, the positive feedback between intensity and photo-polymerization establishes the auto-catalytic behavior. Optical auto-catalysis has been shown to result on spontaneous pattern formation in photopolymers. Hosein and co-workers discovered that optical autocatalysis can also occur in photoreactive polymer blends, and that the process can induce binary phase morphologies with the same pattern as the light profile. Glycolysis consists of the degradation of one molecule of glucose and the overall production of two molecules of ATP. The process is therefore of great importance to the energetics of living cells. The global glycolysis reaction involves glucose, ADP, NAD, pyruvate, ATP, and NADH.
注意到光聚合速率与强度成正比,折射率与分子量成正比,强度与光聚合之间的正反馈建立了自催化行为。光学自催化已被证明是光聚合物自发形成图案的原因。Hosein 和他的合作者发现,光学自催化也可以发生在光反应聚合物共混物中,这一过程可以诱导形成与光型相同的二元相形态。糖酵解是由一个葡萄糖分子的降解和两个 ATP 分子的全部产生组成。因此,这个过程对活细胞的能量学来说是非常重要的。糖酵解反应包括葡萄糖、 ADP、 NAD、丙酮酸、 ATP 和 NADH。. Retrieved 2015-10-15.
{{cite web}}: Check date values in:|archivedate=(help); Invalid|url-status=dead [math]\displaystyle{ \text{polymerization rate} \to \text{molecular weight}/\text{refractive index} \to \text{intensity} }[/math] 文本{分子量}/文本{折射率}到文本{密度}(help); line feed character in|archivedate=at position 12 (help); line feed character in|title=at position 15 (help); line feed character in|url-status=at position 6 (help) Dynamics of the Brusselator - ↑ Peterson, Gabriel. "The Belousov-Zhabotinsky Reaction". Archived from the original on December 31, 2012.
- ↑ 8.0 8.1 8.2 Biria, Saeid; Malley, Phillip P. A.; Kahan, Tara F.; Hosein, Ian D. (2016-11-15). "Optical Autocatalysis Establishes Novel Spatial Dynamics in Phase Separation of Polymer Blends during Photocuring". ACS Macro Letters. 5 (11): 1237–1241. doi:10.1021/acsmacrolett.6b00659.
- ↑ Decker, Christian (1998-02-01). "The use of UV irradiation in polymerization". Polymer International (in English). 45 (2): 133–141. doi:10.1002/(SICI)1097-0126(199802)45:2<133::AID-PI969>3.0.CO;2-F.
- ↑ Askadskii, A. A. (1990). "Influence of crosslinking density on the properties of polymer networks". Polymer Science U.S.S.R. 32 (10): 2061–2069. doi:10.1016/0032-3950(90)90361-9.
- ↑ Burgess, Ian B.; Shimmell, Whitney E.; Saravanamuttu, Kalaichelvi (2007-04-01). "Spontaneous Pattern Formation Due to Modulation Instability of Incoherent White Light in a Photopolymerizable Medium". Journal of the American Chemical Society. 129 (15): 4738–4746. doi:10.1021/ja068967b. ISSN 0002-7863. PMID 17378567.
- ↑ Basker, Dinesh K.; Brook, Michael A.; Saravanamuttu, Kalaichelvi (2015). "Spontaneous Emergence of Nonlinear Light Waves and Self-Inscribed Waveguide Microstructure during the Cationic Polymerization of Epoxides". The Journal of Physical Chemistry C (in English). 119 (35): 20606–20617. doi:10.1021/acs.jpcc.5b07117.
- ↑ Biria, Saeid; Malley, Philip P. A.; Kahan, Tara F.; Hosein, Ian D. (2016-03-03). "Tunable Nonlinear Optical Pattern Formation and Microstructure in Cross-Linking Acrylate Systems during Free-Radical Polymerization". The Journal of Physical Chemistry C. 120 (8): 4517–4528. doi:10.1021/acs.jpcc.5b11377. ISSN 1932-7447.
- ↑ G. Nicolis and Ilya Prigogine (1977). Self-Organization in Nonequilibrium Systems. New York: John Wiley and Sons. ISBN 978-0-471-02401-9.
- ↑ Alfaro-Bittner, K.; Rojas, R.G.; Lafleur, G.; Calvez, S.; Almuneau, G.; Clerc, M.G.; Barbay, S. (22 April 2019). "Modeling the Lateral Wet Oxidation of into Arbitrary Mesa Geometries". Physical Review Applied. 11 (4): 044067. doi:10.1103/PhysRevApplied.11.044067.
- ↑ Soai K, Sato I, Shibata T (2001). "Asymmetric autocatalysis and the origin of chiral homogeneity in organic compounds". The Chemical Record. 1 (4): 321–32. doi:10.1002/tcr.1017. PMID 11893072.
- ↑ Stuart Kauffman (1995). At Home in the Universe: The Search for the Laws of Self-Organization and Complexity. Oxford University Press. ISBN 978-0-19-509599-9. https://archive.org/details/athomeinuniverse00kauf_0.
