“玻尔兹曼方程”的版本间的差异
| 第1行: | 第1行: | ||
| − | 此词条由栗子CUGB翻译整理。 | + | 此词条由栗子CUGB翻译整理。[[File:StairsOfReduction.svg|thumb|The place of the Boltzmann kinetic equation on the stairs of model reduction from microscopic dynamics to macroscopic continuum dynamics (illustration to the content of the book<ref> |
| − | |||
| − | |||
| − | |||
| − | |||
The place of the Boltzmann kinetic equation on the stairs of model reduction from microscopic dynamics to macroscopic continuum dynamics (illustration to the content of the book) | The place of the Boltzmann kinetic equation on the stairs of model reduction from microscopic dynamics to macroscopic continuum dynamics (illustration to the content of the book) | ||
| 第10行: | 第6行: | ||
{{{cite book |last1=Gorban |first1= Alexander N.|last2= Karlin |first2= Ilya V. |date=2005 |title= Invariant Manifolds for Physical and Chemical Kinetics|url= https://www.academia.edu/17378865|url-access=| location= Berlin, Heidelberg |publisher= Springer|series= Lecture Notes in Physics (LNP, vol. 660)| isbn= 978-3-540-22684-0|doi= 10.1007/b98103|via= |quote=}} [https://archive.org/details/gorban-karlin-lnp-2005 Alt URL]</ref>)|链接=Special:FilePath/StairsOfReduction.svg]] | {{{cite book |last1=Gorban |first1= Alexander N.|last2= Karlin |first2= Ilya V. |date=2005 |title= Invariant Manifolds for Physical and Chemical Kinetics|url= https://www.academia.edu/17378865|url-access=| location= Berlin, Heidelberg |publisher= Springer|series= Lecture Notes in Physics (LNP, vol. 660)| isbn= 978-3-540-22684-0|doi= 10.1007/b98103|via= |quote=}} [https://archive.org/details/gorban-karlin-lnp-2005 Alt URL]</ref>)|链接=Special:FilePath/StairsOfReduction.svg]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | The '''Boltzmann equation''' or '''Boltzmann transport equation''' ('''BTE''') describes the statistical behaviour of a [[thermodynamic system]] not in a state of [[Thermodynamic equilibrium|equilibrium]], devised by [[Ludwig Boltzmann]] in 1872.<ref name="Encyclopaediaof">Encyclopaedia of Physics (2nd Edition), R. G. Lerner, G. L. Trigg, VHC publishers, 1991, ISBN (Verlagsgesellschaft) 3-527-26954-1, ISBN (VHC Inc.) 0-89573-752-3.</ref> The classic example of such a system is a fluid with temperature gradients in space causing heat to flow from hotter regions to colder ones, by the random but biased transport of the particles making up that fluid. In the modern literature the term Boltzmann equation is often used in a more general sense, referring to any kinetic equation that describes the change of a macroscopic quantity in a thermodynamic system, such as energy, charge or particle number. | ||
| + | '''玻尔兹曼方程'''或'''玻尔兹曼输运方程(Boltzmann transport equation, BTE)'''是一个描述非热力学平衡状态的热力学系统统计行为的偏微分方程,由'''[[路德维希·玻尔兹曼 Ludwig Edward Boltzmann|路德维希·玻尔兹曼 Ludwig Boltzmann]]'''于1872年提出。<ref name="Encyclopaediaof" /> 这类系统的经典实例是:在空间中具有温度梯度的流体,组成该流体的粒子通过随机但具有偏向性的传输使得热量从较热的区域流向较冷的区域。在现代文献中,玻尔兹曼方程一词通常用于更一般的意义上,指的是描述热力学系统中宏观量变化的任何动力学方程,如能量、电荷或粒子数。 | ||
The equation arises not by analyzing the individual positions and momenta of each particle in the fluid but rather by considering a probability distribution for the position and momentum of a typical particle—that is, the probability that the particle occupies a given very small region of space (mathematically the volume element <math>\mathrm{d}^3 \bf{r}</math>) centered at the position <math>\bf{r}</math>, and has momentum nearly equal to a given momentum vector <math> \bf{p}</math> (thus occupying a very small region of momentum space <math>\mathrm{d}^3 \bf{p}</math>), at an instant of time. | The equation arises not by analyzing the individual positions and momenta of each particle in the fluid but rather by considering a probability distribution for the position and momentum of a typical particle—that is, the probability that the particle occupies a given very small region of space (mathematically the volume element <math>\mathrm{d}^3 \bf{r}</math>) centered at the position <math>\bf{r}</math>, and has momentum nearly equal to a given momentum vector <math> \bf{p}</math> (thus occupying a very small region of momentum space <math>\mathrm{d}^3 \bf{p}</math>), at an instant of time. | ||
| 第88行: | 第75行: | ||
<math> | <math> | ||
| − | + | ||
| − | + | 《数学》 | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | \begin{align} | |
| − | + | ||
| − | + | 开始{ align } | |
| − | + | ||
| − | + | is the number of molecules which ''all'' have positions lying within a volume element <math> d^3\bf{r}</math> about '''r''' and momenta lying within a [[momentum space]] element <math> \mathrm{d}^3\bf{p}</math> about '''p''', at time ''t''.<ref>{{Cite book |last=Huang |first=Kerson |year=1987 |title=Statistical Mechanics |url=https://archive.org/details/statisticalmecha00huan_475 |url-access=limited |location=New York |publisher=Wiley |isbn=978-0-471-81518-1 |page=[https://archive.org/details/statisticalmecha00huan_475/page/n65 53] |edition=Second }}</ref> [[Integration (calculus)|Integrating]] over a region of position space and momentum space gives the total number of particles which have positions and momenta in that region: | |
N & = \int\limits_\mathrm{momenta} \text{d}^3\mathbf{p} \int\limits_\mathrm{positions} \text{d}^3\mathbf{r}\,f (\mathbf{r},\mathbf{p},t) \\[5pt] | N & = \int\limits_\mathrm{momenta} \text{d}^3\mathbf{p} \int\limits_\mathrm{positions} \text{d}^3\mathbf{r}\,f (\mathbf{r},\mathbf{p},t) \\[5pt] | ||
2021年10月24日 (日) 17:43的版本
此词条由栗子CUGB翻译整理。
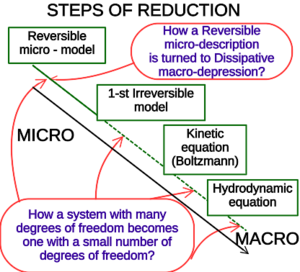
The Boltzmann equation or Boltzmann transport equation (BTE) describes the statistical behaviour of a thermodynamic system not in a state of equilibrium, devised by Ludwig Boltzmann in 1872.[2] The classic example of such a system is a fluid with temperature gradients in space causing heat to flow from hotter regions to colder ones, by the random but biased transport of the particles making up that fluid. In the modern literature the term Boltzmann equation is often used in a more general sense, referring to any kinetic equation that describes the change of a macroscopic quantity in a thermodynamic system, such as energy, charge or particle number.
玻尔兹曼方程或玻尔兹曼输运方程(Boltzmann transport equation, BTE)是一个描述非热力学平衡状态的热力学系统统计行为的偏微分方程,由路德维希·玻尔兹曼 Ludwig Boltzmann于1872年提出。[2] 这类系统的经典实例是:在空间中具有温度梯度的流体,组成该流体的粒子通过随机但具有偏向性的传输使得热量从较热的区域流向较冷的区域。在现代文献中,玻尔兹曼方程一词通常用于更一般的意义上,指的是描述热力学系统中宏观量变化的任何动力学方程,如能量、电荷或粒子数。
The equation arises not by analyzing the individual positions and momenta of each particle in the fluid but rather by considering a probability distribution for the position and momentum of a typical particle—that is, the probability that the particle occupies a given very small region of space (mathematically the volume element [math]\displaystyle{ \mathrm{d}^3 \bf{r} }[/math]) centered at the position [math]\displaystyle{ \bf{r} }[/math], and has momentum nearly equal to a given momentum vector [math]\displaystyle{ \bf{p} }[/math] (thus occupying a very small region of momentum space [math]\displaystyle{ \mathrm{d}^3 \bf{p} }[/math]), at an instant of time.
方程的导出不是通过分析流体中每个粒子的单独位置和动量,而是通过考虑一个典型粒子的位置和动量的概率分布——即粒子某一时刻位于给定位置的小邻域(数学上的体积元[math]\displaystyle{ \mathrm{d}^3 \bf{r} }[/math])、在动量空间占据给定动量矢量[math]\displaystyle{ \bf{p} }[/math]的小邻域([math]\displaystyle{ \mathrm{d}^3 \bf{p} }[/math])的概率。一个给定的非常小的空间区域的概率(数学上是体积元素 < math > mathrm { d } ^ 3 bf { r } </math >) ,动量几乎等于给定的动量矢量 < math > (因此在瞬间占据了一个非常小的动量空间 mathrm { d }3 bf/math >)。
The equation arises not by analyzing the individual positions and momenta of each particle in the fluid but rather by considering a probability distribution for the position and momentum of a typical particle—that is, the probability that the particle occupies a given very small region of space (mathematically the volume element [math]\displaystyle{ \mathrm{d}^3 \bf{r} }[/math]) centered at the position [math]\displaystyle{ \bf{r} }[/math], and has momentum nearly equal to a given momentum vector [math]\displaystyle{ \bf{p} }[/math] (thus occupying a very small region of momentum space [math]\displaystyle{ \mathrm{d}^3 \bf{p} }[/math]), at an instant of time.
The Boltzmann equation can be used to determine how physical quantities change, such as heat energy and momentum, when a fluid is in transport. One may also derive other properties characteristic to fluids such as viscosity, thermal conductivity, and electrical conductivity (by treating the charge carriers in a material as a gas).
玻尔兹曼方程可以用来确定流体在运输过程中物理量如何变化,比如热能和动量。人们还可以推导出流体的其他特性,如粘度、热导率和电导率(通过将材料中的载流子当作气体来处理)。参见对流扩散方程。
The Boltzmann equation can be used to determine how physical quantities change, such as heat energy and momentum, when a fluid is in transport. One may also derive other properties characteristic to fluids such as viscosity, thermal conductivity, and electrical conductivity (by treating the charge carriers in a material as a gas).[2] See also convection–diffusion equation.
The equation is a nonlinear integro-differential equation, and the unknown function in the equation is a probability density function in six-dimensional space of a particle position and momentum. The problem of existence and uniqueness of solutions is still not fully resolved, but some recent results are quite promising.[3][4]
玻尔兹曼方程是一个非线性积分微分方程,方程中的未知函数是位置和动量六维空间中的一个概率密度函数。方程解的存在唯一性仍然是未完全解决的问题,但是近期的一些结果是很有希望的。
Overview 概述
The phase space and density function 相空间和密度函数
The set of all possible positions r and momenta p is called the phase space of the system; in other words a set of three coordinates for each position coordinate x, y, z, and three more for each momentum component px, py, pz. The entire space is 6-dimensional: a point in this space is (r, p) = (x, y, z, px, py, pz), and each coordinate is parameterized by time t. The small volume ("differential volume element") is written
系统所有可能的位置r和动量p的集合称为系统的相空间,每个位置是三个坐标 x,y,z 的集合,另外有三个坐标表示每个动量分量 px, py, pz。整个空间是6维的:空间中的一个点是(r, p) = (x, y, z, px, py, pz),每个坐标由时间 t 参数化。小体积元(“微分体积元”)写作
[math]\displaystyle{ \text{d}^3\mathbf{r}\,\text{d}^3\mathbf{p} = \text{d}x\,\text{d}y\,\text{d}z\,\text{d}p_x\,\text{d}p_y\,\text{d}p_z. }[/math]
< math > text { d } ^ 3 mathbf { r } ,text { d } ^ 3 mathbf { p } = text { d } x,text { d } y,text { d } p _ x,text { d } p _ y,text { d } p _ z.数学
Since the probability of N molecules which all have r and p within [math]\displaystyle{ \mathrm{d}^3\bf{r} }[/math] [math]\displaystyle{ \mathrm{d}^3\bf{p} }[/math] is in question, at the heart of the equation is a quantity f which gives this probability per unit phase-space volume, or probability per unit length cubed per unit momentum cubed, at an instant of time t. This is a probability density function: f(r, p, t), defined so that,
由于 n 分子的概率都有 r 和 p 在 < math > mathrm { d } ^ 3 bf { r } </math > < math > < mathrm { d } ^ 3 bf { p } </math > 存在疑问,方程的核心是一个量 f,它给出了单位相空间体积的概率,或单位长度立方的概率,在一瞬间。这是一个概率密度函数: f (r,p,t) ,定义为,
The set of all possible positions r and momenta p is called the phase space of the system; in other words a set of three coordinates for each position coordinate x, y, z, and three more for each momentum component px, py, pz. The entire space is 6-dimensional: a point in this space is (r, p) = (x, y, z, px, py, pz), and each coordinate is parameterized by time t. The small volume ("differential volume element") is written
- [math]\displaystyle{ \text{d}^3\mathbf{r}\,\text{d}^3\mathbf{p} = \text{d}x\,\text{d}y\,\text{d}z\,\text{d}p_x\,\text{d}p_y\,\text{d}p_z. }[/math]
[math]\displaystyle{ \text{d}N = f (\mathbf{r},\mathbf{p},t)\,\text{d}^3\mathbf{r}\,\text{d}^3\mathbf{p} }[/math]
< math > text { d } n = f (mathbf { r } ,mathbf { p } ,t) ,text { d } ^ 3 mathbf { r } ,text { d } ^ 3 mathbf { p } </math >
Since the probability of N molecules which all have r and p within [math]\displaystyle{ \mathrm{d}^3\bf{r} }[/math] [math]\displaystyle{ \mathrm{d}^3\bf{p} }[/math] is in question, at the heart of the equation is a quantity f which gives this probability per unit phase-space volume, or probability per unit length cubed per unit momentum cubed, at an instant of time t. This is a probability density function: f(r, p, t), defined so that,
is the number of molecules which all have positions lying within a volume element [math]\displaystyle{ d^3\bf{r} }[/math] about r and momenta lying within a momentum space element [math]\displaystyle{ \mathrm{d}^3\bf{p} }[/math] about p, at time t. Integrating over a region of position space and momentum space gives the total number of particles which have positions and momenta in that region:
由于 n 分子的概率都有 r 和 p 在 < math > mathrm { d } ^ 3 bf { r } </math > < math > < mathrm { d } ^ 3 bf { p } </math > 存在疑问,方程的核心是一个量 f,它给出了单位相空间体积的概率,或单位长度立方的概率,在一瞬间。这是一个概率密度函数: f (r,p,t) ,定义为,位于动量空间元素中的 r 和动量的分子数目,在时间 t 上。在位置空间和动量空间的一个区域上积分,得出在该区域中具有位置和动量的粒子总数:
- [math]\displaystyle{ \text{d}N = f (\mathbf{r},\mathbf{p},t)\,\text{d}^3\mathbf{r}\,\text{d}^3\mathbf{p} }[/math]
[math]\displaystyle{ 《数学》 \begin{align} 开始{ align } is the number of molecules which ''all'' have positions lying within a volume element \lt math\gt d^3\bf{r} }[/math] about r and momenta lying within a momentum space element [math]\displaystyle{ \mathrm{d}^3\bf{p} }[/math] about p, at time t.[5] Integrating over a region of position space and momentum space gives the total number of particles which have positions and momenta in that region:
N & = \int\limits_\mathrm{momenta} \text{d}^3\mathbf{p} \int\limits_\mathrm{positions} \text{d}^3\mathbf{r}\,f (\mathbf{r},\mathbf{p},t) \\[5pt]
N & = int limits _ mathrm { momenta } text { d } ^ 3 mathbf { p } int limits _ mathrm { positions } text { d } ^ 3 mathbf { r } ,f (mathbf { r } ,mathbf { p } ,t)[5 pt ]
& = \iiint\limits_\mathrm{momenta} \quad \iiint\limits_\mathrm{positions} f(x,y,z,p_x,p_y,p_z,t) \, \text{d}x \, \text{d}y \, \text{d}z \, \text{d}p_x \, \text{d}p_y \, \text{d}p_z
限制,限制,限制,限制,限制,限制,限制,限制,限制
- [math]\displaystyle{ \end{align} 结束{ align } \begin{align} }[/math]
数学
N & = \int\limits_\mathrm{momenta} \text{d}^3\mathbf{p} \int\limits_\mathrm{positions} \text{d}^3\mathbf{r}\,f (\mathbf{r},\mathbf{p},t) \\[5pt]
& = \iiint\limits_\mathrm{momenta} \quad \iiint\limits_\mathrm{positions} f(x,y,z,p_x,p_y,p_z,t) \, \text{d}x \, \text{d}y \, \text{d}z \, \text{d}p_x \, \text{d}p_y \, \text{d}p_z
which is a 6-fold integral. While f is associated with a number of particles, the phase space is for one-particle (not all of them, which is usually the case with deterministic many-body systems), since only one r and p is in question. It is not part of the analysis to use r1, p1 for particle 1, r2, p2 for particle 2, etc. up to rN, pN for particle N.
这是一个6重积分。虽然 f 与许多粒子相关联,但相空间是单粒子的(不是所有粒子,通常是确定性多体系统的情况) ,因为只有一个 r 和 p 存在问题。用 r < sub > 1 、 p < sub > 1 表示粒子1、 r < sub > 2 、 p < sub > 2 表示粒子2等不属于分析范围。粒子 n 可达 r < sub > n ,p < sub > n 。
\end{align}
</math>
It is assumed the particles in the system are identical (so each has an identical mass m). For a mixture of more than one chemical species, one distribution is needed for each, see below.
假设系统中的粒子是相同的(因此每个粒子的质量都是相同的)。对于一种以上化学物质的混合物,需要对每种物质进行一次分配,见下文。
which is a 6-fold integral. While f is associated with a number of particles, the phase space is for one-particle (not all of them, which is usually the case with deterministic many-body systems), since only one r and p is in question. It is not part of the analysis to use r1, p1 for particle 1, r2, p2 for particle 2, etc. up to rN, pN for particle N.
It is assumed the particles in the system are identical (so each has an identical mass m). For a mixture of more than one chemical species, one distribution is needed for each, see below.
The general equation can then be written as
一般的方程式可以写成
Principal statement
[math]\displaystyle{ 《数学》 \frac{df}{dt} = 1 = = = = = = The general equation can then be written as\lt ref name="McGrawHill"\gt McGraw Hill Encyclopaedia of Physics (2nd Edition), C. B. Parker, 1994, {{ISBN|0-07-051400-3}}.\lt /ref\gt \left(\frac{\partial f}{\partial t}\right)_\text{force} + 左(frac { partial f }{ partial t } right) _ text { force } + \left(\frac{\partial f}{\partial t}\right)_\text{diff} + 左(frac { partial f }{ partial t } right) _ text { diff } + :\lt math\gt \left(\frac{\partial f}{\partial t}\right)_\text{coll}, 左(frac { partial f }{ partial t } right) _ text { coll } , \frac{df}{dt} = }[/math]
数学
\left(\frac{\partial f}{\partial t}\right)_\text{force} +
\left(\frac{\partial f}{\partial t}\right)_\text{diff} +
where the "force" term corresponds to the forces exerted on the particles by an external influence (not by the particles themselves), the "diff" term represents the diffusion of particles, and "coll" is the collision term – accounting for the forces acting between particles in collisions. Expressions for each term on the right side are provided below. The assumption in the BGK approximation is that the effect of molecular collisions is to force a non-equilibrium distribution function at a point in physical space back to a Maxwellian equilibrium distribution function and that the rate at which this occurs is proportional to the molecular collision frequency. The Boltzmann equation is therefore modified to the BGK form:
如果“力”这个术语对应于外部影响(而不是粒子本身)施加在粒子上的力,“ diff”这个术语代表粒子的扩散,“ coll”是碰撞术语——解释粒子之间在碰撞中的作用力。下面提供了右边每个术语的表达式。BGK 近似中的假设是,分子碰撞的效果是迫使物理空间中某一点的非平衡分布函数回到马克斯韦尔平衡分布函数,而这种情况发生的速率与分子碰撞频率成正比。因此,《玻尔兹曼方程修改为 BGK 格式:
\left(\frac{\partial f}{\partial t}\right)_\text{coll},
</math>
[math]\displaystyle{ \frac{\partial f}{\partial t} + \frac{\mathbf{p}}{m}\cdot\nabla f + \mathbf{F} \cdot \frac{\partial f}{\partial \mathbf{p}} = \nu (f_0 - f), }[/math]
[数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学][部分数学]
where the "force" term corresponds to the forces exerted on the particles by an external influence (not by the particles themselves), the "diff" term represents the diffusion of particles, and "coll" is the collision term – accounting for the forces acting between particles in collisions. Expressions for each term on the right side are provided below.[6]
where [math]\displaystyle{ \nu }[/math] is the molecular collision frequency, and [math]\displaystyle{ f_0 }[/math] is the local Maxwellian distribution function given the gas temperature at this point in space.
其中“ nu”是分子碰撞频率,而“ math”是给定空间此点气体温度的局部马克斯韦尔分布函数。
Note that some authors use the particle velocity v instead of momentum p; they are related in the definition of momentum by p = mv.
The force and diffusion terms
For a mixture of chemical species labelled by indices i = 1, 2, 3, ..., n the equation for species i is For a fluid consisting of only one kind of particle, the number density n is given by
对于以指数 i = 1,2,3,... ,n 标记的化学物种混合物,物种 i 的方程是: 对于只包含一种粒子的流体,数密度 n 由
[math]\displaystyle{ n = \int f \,d^3p. }[/math]
[ math ] n = int f,d ^ 3p
Consider particles described by f, each experiencing an external force F not due to other particles (see the collision term for the latter treatment).
The average value of any function A is
任何函数 a 的平均值都是
Suppose at time t some number of particles all have position r within element [math]\displaystyle{ d^3\bf{r} }[/math] and momentum p within [math]\displaystyle{ d^3\bf{p} }[/math]. If a force F instantly acts on each particle, then at time t + Δt their position will be r + Δr = r + pΔt/m and momentum p + Δp = p + FΔt. Then, in the absence of collisions, f must satisfy
[math]\displaystyle{ \langle A \rangle = \frac 1 n \int A f \,d^3p. }[/math]
A rangle = frac 1n int a f,d ^ 3p
- [math]\displaystyle{ Since the conservation equations involve tensors, the Einstein summation convention will be used where repeated indices in a product indicate summation over those indices. Thus \lt math\gt \mathbf{x} \mapsto x_i }[/math] and [math]\displaystyle{ \mathbf{p} \mapsto p_i = m w_i }[/math], where [math]\displaystyle{ w_i }[/math] is the particle velocity vector. Define [math]\displaystyle{ A(p_i) }[/math] as some function of momentum [math]\displaystyle{ p_i }[/math] only, which is conserved in a collision. Assume also that the force [math]\displaystyle{ F_i }[/math] is a function of position only, and that f is zero for [math]\displaystyle{ p_i \to \pm\infty }[/math]. Multiplying the Boltzmann equation by A and integrating over momentum yields four terms, which, using integration by parts, can be expressed as
由于守恒方程涉及张量,爱因斯坦总和约定将用于重复索引在一个积表明总和超过这些索引。因此,mathbf { x }映射到 x i </math > 和 < math > mathbf { p }映射到 p i = m w i </math > ,其中 < math > w i </math > 是粒子速度矢量。定义 a (p _ i) </math > 为动量 < math > p _ i </math > 的某个函数,它在碰撞中是守恒的。还假设力 < math > f _ i </math > 是位置的函数,而且 f 对 < math > p _ i 到 pm </math > 是0。用玻尔兹曼方程乘以 a,再加上动量积分得到4个术语,用部分积分可以表示为
f \left (\mathbf{r}+\frac{\mathbf{p}}{m} \, \Delta t,\mathbf{p}+\mathbf{F} \, \Delta t, t+\Delta t \right )\,d^3\mathbf{r}\,d^3\mathbf{p} = f(\mathbf{r}, \mathbf{p},t) \, d^3\mathbf{r} \, d^3\mathbf{p}
</math>
[math]\displaystyle{ \int A \frac{\partial f}{\partial t} \,d^3p = \frac{\partial }{\partial t} (n \langle A \rangle), }[/math]
(n langle a rangle) ,</math >
Note that we have used the fact that the phase space volume element [math]\displaystyle{ d^3\bf{r} }[/math] [math]\displaystyle{ d^3\bf{p} }[/math] is constant, which can be shown using Hamilton's equations (see the discussion under Liouville's theorem). However, since collisions do occur, the particle density in the phase-space volume [math]\displaystyle{ d^3\bf{r} }[/math] '[math]\displaystyle{ d^3\bf{p} }[/math] changes, so
[math]\displaystyle{ \int \frac{p_j A}{m}\frac{\partial f}{\partial x_j} \,d^3p = \frac{1}{m}\frac{\partial}{\partial x_j}(n\langle A p_j \rangle), }[/math]
[数学][数学][数学][数学][数学][数学]
-
{{{2}}}
({{{3}}})
{m}\Delta t,\mathbf{p} + \mathbf{F}\Delta t, t+\Delta t \right)d^3\mathbf{r}d^3\mathbf{p} - f(\mathbf{r}, \mathbf{p}, t) \, d^3\mathbf{r} \, d^3\mathbf{p} \\[5pt]
& = \Delta f \, d^3\mathbf{r} \, d^3\mathbf{p}
where the last term is zero, since A is conserved in a collision. Letting [math]\displaystyle{ A = m }[/math], the mass of the particle, the integrated Boltzmann equation becomes the conservation of mass equation: including the formation of the light elements in Big Bang nucleosynthesis, the production of dark matter and baryogenesis. It is not a priori clear that the state of a quantum system can be characterized by a classical phase space density f. However, for a wide class of applications a well-defined generalization of f exists which is the solution of an effective Boltzmann equation that can be derived from first principles of quantum field theory.
最后一项是零,因为 a 在碰撞中守恒。让粒子的质量,积分玻尔兹曼方程成为质量守恒方程: 包括太初核合成中轻元素的形成,暗物质的产生和重子形成。量子系统的状态是否可以用经典的相空间密度 f 来表示,这一点先验上并不清楚。然而,对于广泛的应用来说,f 的一个定义明确的推广是存在的,它是一个有效的拥有属性玻尔兹曼方程的解,可以从量子场论的第一原理中推导出来。
\end{align}</math>
|1}}
where Δf is the total change in f. Dividing (1) by [math]\displaystyle{ d^3\bf{r} }[/math] [math]\displaystyle{ d^3\bf{p} }[/math] Δt and taking the limits Δt → 0 and Δf → 0, we have
The Boltzmann equation is of use in galactic dynamics. A galaxy, under certain assumptions, may be approximated as a continuous fluid; its mass distribution is then represented by f; in galaxies, physical collisions between the stars are very rare, and the effect of gravitational collisions can be neglected for times far longer than the age of the universe.
玻尔兹曼方程星云在银河系动力学中有用。在某些假设下,一个星系可以近似为一个连续的流体; 它的质量分布用 f 来表示; 在星系中,恒星之间的物理碰撞是非常罕见的,重力碰撞的影响可以忽略倍于宇宙年龄的时间。
-
Its generalization in general relativity. is
它在广义相对论的推广。是
[math]\displaystyle{ \frac{d f}{d t} = \left(\frac{\partial f}{\partial t} \right)_\mathrm{coll} }[/math]
(2)
[math]\displaystyle{ \hat{\mathbf{L}}_\mathrm{GR}=p^\alpha\frac{\partial}{\partial x^\alpha} - \Gamma^\alpha{}_{\beta\gamma}p^\beta p^\gamma\frac{\partial}{\partial p^\alpha}, }[/math]
{ mathbf { l } mathrm { GR } = p ^ alpha frac { partial }{ x ^ alpha }-Gamma ^ alpha {}{ beta Gamma } p ^ p ^ frac { partial }{ partial p ^ alpha } ,</math >
The total differential of f is:
where Γαβγ is the Christoffel symbol of the second kind (this assumes there are no external forces, so that particles move along geodesics in the absence of collisions), with the important subtlety that the density is a function in mixed contravariant-covariant (xi, pi) phase space as opposed to fully contravariant (xi, pi) phase space.
其中 γ < sup > α < βγ 是第二类 Christoffel 符号(假设没有外力,因此粒子在没有碰撞的情况下沿测地线运动) ,其中重要的微妙之处在于密度是混合逆变-协变(x < sup > i ,p < sub > i )相空间中的函数,而不是完全逆变(x < sup > i ,p )相空间中的函数。
-
In physical cosmology the fully covariant approach has been used to study the cosmic microwave background radiation. More generically the study of processes in the early universe often attempt to take into account the effects of quantum mechanics and general relativity. this analytical approach provides insight, but is not generally usable in practical problems.
20世纪90年代物理宇宙学,完全协变方法被用于研究宇宙微波背景辐射。更一般地说,对早期宇宙过程的研究往往试图考虑量子力学和广义相对论的影响。这种分析方法提供了洞察力,但在实际问题中通常不能使用。
[math]\displaystyle{ \begin{align} d f & = \frac{\partial f}{\partial t} \, dt Instead, numerical methods (including finite elements) are generally used to find approximate solutions to the various forms of the Boltzmann equation. Example applications range from hypersonic aerodynamics in rarefied gas flows to plasma flows. 相反,数值方法(包括有限元)通常用于寻找各种形式的玻尔兹曼方程的近似解。示例应用范围从稀薄气流中的高超音速空气动力学到等离子流。 +\left(\frac{\partial f}{\partial x} \, dx +\frac{\partial f}{\partial y} \, dy Close to local equilibrium, solution of the Boltzmann equation can be represented by an asymptotic expansion in powers of Knudsen number (the Chapman-Enskog expansion). The first two terms of this expansion give the Euler equations and the Navier-Stokes equations. The higher terms have singularities. The problem of developing mathematically the limiting processes, which lead from the atomistic view (represented by Boltzmann's equation) to the laws of motion of continua, is an important part of Hilbert's sixth problem. 在接近局部均衡的情况下,玻尔兹曼方程的解可以用一个克努森数的渐近展开表示(Chapman-Enskog 展开式)。这个展开式的前两项给出了欧拉方程和纳维-斯托克斯方程。较高的项有奇点。从原子观点(以玻尔兹曼方程为代表)到连续统运动定律的极限过程的数学发展问题,是希尔伯特第六个问题的重要组成部分。 +\frac{\partial f}{\partial z} \, dz \right) +\left(\frac{\partial f}{\partial p_x} \, dp_x +\frac{\partial f}{\partial p_y} \, dp_y +\frac{\partial f}{\partial p_z} \, dp_z \lt !--*BGK equation \lt ! -- * BGK 方程 \right)\\[5pt] & = \frac{\partial f}{\partial t}dt +\nabla f \cdot d\mathbf{r} + \frac{\partial f}{\partial \mathbf{p}}\cdot d\mathbf{p} \\[5pt] & = \frac{\partial f}{\partial t}dt +\nabla f \cdot \frac{\mathbf{p}}{m}dt + \frac{\partial f}{\partial \mathbf{p}}\cdot \mathbf{F} \, dt \end{align} }[/math]
(3)
where ∇ is the gradient operator, · is the dot product,
- [math]\displaystyle{ \frac{\partial f}{\partial \mathbf{p}} = \mathbf{\hat{e}}_x\frac{\partial f}{\partial p_x} + \mathbf{\hat{e}}_y\frac{\partial f}{\partial p_y} + \mathbf{\hat{e}}_z \frac{\partial f}{\partial p_z}= \nabla_\mathbf{p}f }[/math]
is a shorthand for the momentum analogue of ∇, and êx, êy, êz are Cartesian unit vectors.
Final statement
Dividing (3) by dt and substituting into (2) gives:
| last1= Harris
1 = Harris
| first1= Stewart
1 = Stewart
- [math]\displaystyle{ \frac{\partial f}{\partial t} + \frac{\mathbf{p}}{m}\cdot\nabla f + \mathbf{F} \cdot \frac{\partial f}{\partial \mathbf{p}} = \left(\frac{\partial f}{\partial t} \right)_\mathrm{coll} }[/math]
|author1-link=
1-link =
| title= An introduction to the theory of the Boltzmann equation | publisher=Dover Books|pages=221 | year= 1971 | isbn=978-0-486-43831-3|url=https://books.google.com/books?id=KfYK1lyq3VYC}}. Very inexpensive introduction to the modern framework (starting from a formal deduction from Liouville and the Bogoliubov–Born–Green–Kirkwood–Yvon hierarchy (BBGKY) in which the Boltzmann equation is placed). Most statistical mechanics textbooks like Huang still treat the topic using Boltzmann's original arguments. To derive the equation, these books use a heuristic explanation that does not bring out the range of validity and the characteristic assumptions that distinguish Boltzmann's from other transport equations like Fokker–Planck or Landau equations.
| title = 玻尔兹曼方程理论导论 | 出版商 = Dover Books | pages = 221 | year = 1971 | isbn = 978-0-486-43831-3 | url = https://Books.google.com/Books?id=kfyk1lyq3vyc }。非常便宜的现代框架介绍(从一个正式的推论从 Liouville 和 Bogoliubov-Born-Green-Kirkwood-伊冯等级(BBGKY)的玻尔兹曼方程是放置)。大多数统计力学的教科书,比如 Huang,仍然使用 Boltzmann 的原始论点来处理这个话题。为了推导这个方程,这些书使用了一种启发式的解释,这种解释没有提出波尔兹曼方程与其他传输方程(如福克-普朗克方程或兰道方程)区别开来的有效性范围和特征性假设。
In this context, F(r, t) is the force field acting on the particles in the fluid, and m is the mass of the particles. The term on the right hand side is added to describe the effect of collisions between particles; if it is zero then the particles do not collide. The collisionless Boltzmann equation, where individual collisions are replaced with long-range aggregated interactions, e.g. Coulomb interactions, is often called the Vlasov equation.
This equation is more useful than the principal one above, yet still incomplete, since f cannot be solved unless the collision term in f is known. This term cannot be found as easily or generally as the others – it is a statistical term representing the particle collisions, and requires knowledge of the statistics the particles obey, like the Maxwell–Boltzmann, Fermi–Dirac or Bose–Einstein distributions.
| last1= Arkeryd
| last1= Arkeryd
The collision term (Stosszahlansatz) and molecular chaos
| first1= Leif
1 = Leif
|author1-link= Leif Arkeryd
1-link = Leif Arkeryd
Two-body collision term
| title= On the Boltzmann equation part II: The full initial value problem | journal= Arch. Rational Mech. Anal. | volume= 45 | issue= 1
第二部分: 完整的初始值问题 | 日志 = 玻尔兹曼方程。Rational Mech.肛交。45 | issue = 1
A key insight applied by Boltzmann was to determine the collision term resulting solely from two-body collisions between particles that are assumed to be uncorrelated prior to the collision. This assumption was referred to by Boltzmann as the "脚本错误:没有“lang”这个模块。" and is also known as the "molecular chaos assumption". Under this assumption the collision term can be written as a momentum-space integral over the product of one-particle distribution functions:[2]
| pages= 17–34 | year= 1972 | doi = 10.1007/BF00253393 | bibcode = 1972ArRMA..45...17A | s2cid= 119481100
17-34 | year = 1972 | doi = 10.1007/BF00253393 | bibcode = 1972ArRMA. 45... 17A | s2cid = 119481100
- [math]\displaystyle{ }} }} \left(\frac{\partial f}{\partial t}\right)_\text{coll} = \iint gI(g, \Omega)[f(\mathbf{r},\mathbf{p'}_A, t) f(\mathbf{r},\mathbf{p'}_B,t) - f(\mathbf{r},\mathbf{p}_A,t) f(\mathbf{r},\mathbf{p}_B,t)] \,d\Omega \,d^3\mathbf{p}_A \,d^3\mathbf{p}_B, }[/math]
where pA and pB are the momenta of any two particles (labeled as A and B for convenience) before a collision, p′A and p′B are the momenta after the collision,
- [math]\displaystyle{ g = |\mathbf{p}_B - \mathbf{p}_A| = |\mathbf{p'}_B - \mathbf{p'}_A| }[/math]
is the magnitude of the relative momenta (see relative velocity for more on this concept), and I(g, Ω) is the differential cross section of the collision, in which the relative momenta of the colliding particles turns through an angle θ into the element of the solid angle dΩ, due to the collision.
Category:Partial differential equations
类别: 偏微分方程
Category:Statistical mechanics
类别: 统计力学
Simplifications to the collision term
Category:Transport phenomena
类别: 运输现象
Since much of the challenge in solving the Boltzmann equation originates with the complex collision term, attempts have been made to "model" and simplify the collision term. The best known model equation is due to Bhatnagar, Gross and Krook.[7] The assumption in the BGK approximation is that the effect of molecular collisions is to force a non-equilibrium distribution function at a point in physical space back to a Maxwellian equilibrium distribution function and that the rate at which this occurs is proportional to the molecular collision frequency. The Boltzmann equation is therefore modified to the BGK form:
Category:Equations of physics
类别: 物理方程
Equation
方程式
- [math]\displaystyle{ \frac{\partial f}{\partial t} + \frac{\mathbf{p}}{m}\cdot\nabla f + \mathbf{F} \cdot \frac{\partial f}{\partial \mathbf{p}} = \nu (f_0 - f), }[/math]
Category:1872 in science
类别: 1872年的科学
Category:1872 in Germany
类别: 1872年在德国
This page was moved from wikipedia:en:Boltzmann equation. Its edit history can be viewed at 玻尔兹曼方程/edithistory
- ↑ The place of the Boltzmann kinetic equation on the stairs of model reduction from microscopic dynamics to macroscopic continuum dynamics (illustration to the content of the book) 玻耳兹曼动力学方程在从微观动力学到宏观连续动力学的模型简化阶梯上的位置(本书内容的说明) {Gorban, Alexander N.; Karlin, Ilya V. (2005). Invariant Manifolds for Physical and Chemical Kinetics. Lecture Notes in Physics (LNP, vol. 660). Berlin, Heidelberg: Springer. doi:10.1007/b98103. ISBN 978-3-540-22684-0. https://www.academia.edu/17378865. Alt URL
- ↑ 2.0 2.1 2.2 2.3 Encyclopaedia of Physics (2nd Edition), R. G. Lerner, G. L. Trigg, VHC publishers, 1991, ISBN (Verlagsgesellschaft) 3-527-26954-1, ISBN (VHC Inc.) 0-89573-752-3.
- ↑ DiPerna, R. J.; Lions, P.-L. (1989). "On the Cauchy problem for Boltzmann equations: global existence and weak stability". Ann. of Math. 2. 130 (2): 321–366. doi:10.2307/1971423. JSTOR 1971423.
- ↑ Philip T. Gressman & Robert M. Strain (2010). "Global classical solutions of the Boltzmann equation with long-range interactions". Proceedings of the National Academy of Sciences. 107 (13): 5744–5749. arXiv:1002.3639. Bibcode:2010PNAS..107.5744G. doi:10.1073/pnas.1001185107. PMC 2851887. PMID 20231489.
- ↑ Huang, Kerson (1987). Statistical Mechanics (Second ed.). New York: Wiley. p. 53. ISBN 978-0-471-81518-1. https://archive.org/details/statisticalmecha00huan_475.
- ↑ 引用错误:无效
<ref>标签;未给name属性为McGrawHill的引用提供文字 - ↑ Bhatnagar, P. L.; Gross, E. P.; Krook, M. (1954-05-01). "A Model for Collision Processes in Gases. I. Small Amplitude Processes in Charged and Neutral One-Component Systems". Physical Review. 94 (3): 511–525. Bibcode:1954PhRv...94..511B. doi:10.1103/PhysRev.94.511.