自由度
此词条暂由彩云小译翻译,翻译字数共1459,未经人工整理和审校,带来阅读不便,请见谅。
In physics and chemistry, a degree of freedom is an independent physical parameter in the formal description of the state of a physical system. The set of all states of a system is known as the system's phase space, and the degrees of freedom of the system are the dimensions of the phase space.
In physics and chemistry, a degree of freedom is an independent physical parameter in the formal description of the state of a physical system. The set of all states of a system is known as the system's phase space, and the degrees of freedom of the system are the dimensions of the phase space.
在物理学和化学中,自由度是物理系统状态形式描述中一个独立的物理参数。系统所有状态的集合称为系统的相空间,系统的自由度称为系统的相空间的维数。
The location of a particle in three-dimensional space requires three position coordinates. Similarly, the direction and speed at which a particle moves can be described in terms of three velocity components, each in reference to the three dimensions of space. If the time evolution of the system is deterministic, where the state at one instant uniquely determines its past and future position and velocity as a function of time, such a system has six degrees of freedom.[citation needed] If the motion of the particle is constrained to a lower number of dimensions – for example, the particle must move along a wire or on a fixed surface – then the system has fewer than six degrees of freedom. On the other hand, a system with an extended object that can rotate or vibrate can have more than six degrees of freedom.
The location of a particle in three-dimensional space requires three position coordinates. Similarly, the direction and speed at which a particle moves can be described in terms of three velocity components, each in reference to the three dimensions of space. If the time evolution of the system is deterministic, where the state at one instant uniquely determines its past and future position and velocity as a function of time, such a system has six degrees of freedom. If the motion of the particle is constrained to a lower number of dimensions – for example, the particle must move along a wire or on a fixed surface – then the system has fewer than six degrees of freedom. On the other hand, a system with an extended object that can rotate or vibrate can have more than six degrees of freedom.
粒子在三维空间中的位置需要3个位置坐标。同样,粒子运动的方向和速度可以用三个速度分量来描述,每个分量都参照空间的三个维度。如果系统的时间演化是确定的,状态在某一时刻唯一地决定了它的过去和未来的位置和速度作为时间的函数,这样的系统有六个自由度。如果粒子的运动被限制在较低的维数上——例如,粒子必须沿着导线或在固定的表面上运动——那么系统的自由度就少于6度。另一方面,一个系统与一个扩展的对象,可以旋转或振动可以有六个以上的自由度。
In classical mechanics, the state of a point particle at any given time is often described with position and velocity coordinates in the Lagrangian formalism, or with position and momentum coordinates in the Hamiltonian formalism.
In classical mechanics, the state of a point particle at any given time is often described with position and velocity coordinates in the Lagrangian formalism, or with position and momentum coordinates in the Hamiltonian formalism.
在经典力学中,一个点粒子在任意给定时间的状态通常用拉格朗日公式中的位置和速度坐标来描述,或者用哈密顿公式中的位置和动量坐标来描述。
In statistical mechanics, a degree of freedom is a single scalar number describing the microstate of a system.[1] The specification of all microstates of a system is a point in the system's phase space.
In statistical mechanics, a degree of freedom is a single scalar number describing the microstate of a system. The specification of all microstates of a system is a point in the system's phase space.
在统计力学中,一个自由度是描述系统微观状态的单个标量数。一个系统的所有微观状态的描述是系统相空间中的一个点。
In the 3D ideal chain model in chemistry, two angles are necessary to describe the orientation of each monomer.
In the 3D ideal chain model in chemistry, two angles are necessary to describe the orientation of each monomer.
在化学三维理想链模型中,每个单体的取向需要用两个角度来描述。
It is often useful to specify quadratic degrees of freedom. These are degrees of freedom that contribute in a quadratic function to the energy of the system.
It is often useful to specify quadratic degrees of freedom. These are degrees of freedom that contribute in a quadratic function to the energy of the system.
指定二次自由度常常是有用的。这些自由度以二次函数的形式,对系统的能量做出贡献。
Gas molecules
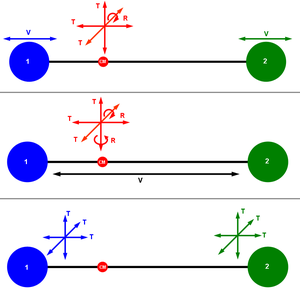
Different ways of visualizing the 6 degrees of freedom of a diatomic molecule. (CM: center of mass of the system, T: translational motion, R: rotational motion, V: vibrational motion.)
用不同的方式可视化双原子分子的6个自由度。(CM: [系统的质心,t: 平动运动,r: 旋转轴,v: 振动运动]]
In three-dimensional space, three degrees of freedom are associated with the movement of a particle. A diatomic gas molecule has 6 degrees of freedom模板:Dubious. This set may be decomposed in terms of translations, rotations, and vibrations of the molecule. The center of mass motion of the entire molecule accounts for 3 degrees of freedom. In addition, the molecule has two rotational degrees of motion and one模板:Dubious vibrational mode. The rotations occur around the two axes perpendicular to the line between the two atoms. The rotation around the atom–atom bond is not a physical rotation模板:Dubious. This yields, for a diatomic molecule, a decomposition of:
In three-dimensional space, three degrees of freedom are associated with the movement of a particle. A diatomic gas molecule has 6 degrees of freedom. This set may be decomposed in terms of translations, rotations, and vibrations of the molecule. The center of mass motion of the entire molecule accounts for 3 degrees of freedom. In addition, the molecule has two rotational degrees of motion and one vibrational mode. The rotations occur around the two axes perpendicular to the line between the two atoms. The rotation around the atom–atom bond is not a physical rotation. This yields, for a diatomic molecule, a decomposition of:
在三维空间中,3个自由度与一个粒子的运动有关。一个双原子气体分子有6个自由度。这个集合可以根据分子的平动、转动和振动来分解。整个分子的质心运动占3个自由度。此外,分子有两个转动度和一个振动模式。旋转发生在两个轴与两个原子之间的直线垂直的周围。原子-原子键的旋转不是物理旋转。这样,在一个双原子分子的时间里,产生了一个分解过程:
- [math]\displaystyle{ N = 6 = 3 + 2 + 1. }[/math]
[math]\displaystyle{ N = 6 = 3 + 2 + 1. }[/math]
6 = 3 + 2 + 1
For a general, non-linear molecule, all 3 rotational degrees of freedom are considered, resulting in the decomposition:
For a general, non-linear molecule, all 3 rotational degrees of freedom are considered, resulting in the decomposition:
对于一般的非线性分子,考虑了所有3个转动自由度,导致分解:
- [math]\displaystyle{ 3N = 3 + 3 + (3N - 6) }[/math]
[math]\displaystyle{ 3N = 3 + 3 + (3N - 6) }[/math]
3N = 3 + 3 + (3N-6) </math >
which means that an N-atom molecule has 3N − 6 vibrational degrees of freedom for N > 2. In special cases, such as adsorbed large molecules, the rotational degrees of freedom can be limited to only one.[2]
which means that an -atom molecule has vibrational degrees of freedom for . In special cases, such as adsorbed large molecules, the rotational degrees of freedom can be limited to only one.
这意味着一个原子分子的振动自由度。在特殊情况下,如吸附大分子,转动自由度可以限制为只有一个。
As defined above one can also count degrees of freedom using the minimum number of coordinates required to specify a position. This is done as follows:
As defined above one can also count degrees of freedom using the minimum number of coordinates required to specify a position. This is done as follows:
根据上面的定义,还可以使用指定位置所需的最小坐标数来计算自由度。具体做法如下:
- For a single particle we need 2 coordinates in a 2-D plane to specify its position and 3 coordinates in 3-D space. Thus its degree of freedom in a 3-D space is 3.
For a single particle we need 2 coordinates in a 2-D plane to specify its position and 3 coordinates in 3-D space. Thus its degree of freedom in a 3-D space is 3.
对于单个粒子,我们需要二维平面中的两个坐标来指定它的位置,以及三维空间中的三个坐标。因此,它在三维空间中的自由度为3。
- For a body consisting of 2 particles (ex. a diatomic molecule) in a 3-D space with constant distance between them (let's say d) we can show (below) its degrees of freedom to be 5.
For a body consisting of 2 particles (ex. a diatomic molecule) in a 3-D space with constant distance between them (let's say d) we can show (below) its degrees of freedom to be 5.
对于由两个粒子组成的物体(例如。在一个三维空间中,两个双原子分子之间的距离不变(比如说 d) ,我们可以显示它的自由度为5。
Let's say one particle in this body has coordinate (x1, y1, z1) and the other has coordinate (x2, y2, z2) with z2 unknown. Application of the formula for distance between two coordinates
Let's say one particle in this body has coordinate and the other has coordinate with unknown. Application of the formula for distance between two coordinates
假设这个物体中的一个粒子具有坐标,而另一个粒子具有未知坐标。两坐标间距公式的应用
- [math]\displaystyle{ d=\sqrt{(x_2-x_1)^2+(y_2-y_1)^2+(z_2-z_1)^2} }[/math]
[math]\displaystyle{ d=\sqrt{(x_2-x_1)^2+(y_2-y_1)^2+(z_2-z_1)^2} }[/math]
[ math > d = sqrt {(x _ 2-x _ 1) ^ 2 + (y _ 2-y _ 1) ^ 2 + (z _ 2-z _ 1) ^ 2][ math >
results in one equation with one unknown, in which we can solve for z2.
results in one equation with one unknown, in which we can solve for .
结果是一个方程有一个未知数,在这个方程中我们可以解出。
One of x1, x2, y1, y2, z1, or z2 can be unknown.
One of , , , , , or can be unknown.
其中之一,,,,,或者可能是未知的。
Contrary to the classical equipartition theorem, at room temperature, the vibrational motion of molecules typically makes negligible contributions to the heat capacity. This is because these degrees of freedom are frozen because the spacing between the energy eigenvalues exceeds the energy corresponding to ambient temperatures (kBT). In the following table such degrees of freedom are disregarded because of their low effect on total energy. Then only the translational and rotational degrees of freedom contribute, in equal amounts, to the heat capacity ratio. This is why γ=模板:Sfrac for monatomic gases and γ=模板:Sfrac for diatomic gases at room temperature.
Contrary to the classical equipartition theorem, at room temperature, the vibrational motion of molecules typically makes negligible contributions to the heat capacity. This is because these degrees of freedom are frozen because the spacing between the energy eigenvalues exceeds the energy corresponding to ambient temperatures (). In the following table such degrees of freedom are disregarded because of their low effect on total energy. Then only the translational and rotational degrees of freedom contribute, in equal amounts, to the heat capacity ratio. This is why =}} for monatomic gases and =}} for diatomic gases at room temperature.
与经典的能量均分定理相反,在室温下,分子的振动运动对热容的贡献可以忽略不计。这是因为这些自由度是冻结的,因为能量本征值之间的间距超过了对应于环境温度的能量()。在下表中,这种自由度不予考虑,因为它们对总能量的影响很小。那么,只有平动自由度和转动自由度,以相等的数量贡献于热容比。这就是为什么对于单原子气体和对于室温下的双原子气体。
However, at very high temperatures, on the order of the vibrational temperature (Θvib), vibrational motion cannot be neglected.
However, at very high temperatures, on the order of the vibrational temperature (Θvib), vibrational motion cannot be neglected.
然而,在非常高的温度下,按振动温度(θ < sub > vib )的顺序,振动运动是不可忽略的。
Vibrational temperatures are between 103 K and 104 K.[1]
Vibrational temperatures are between 103 K and 104 K.
振动温度在10 < sup > 3 k 和10 < sup > 4 k 之间。
| Monatomic | Monatomic | 单原子的 | Linear molecules | Linear molecules | 线性分子 | Non-linear molecules | Non-linear molecules | 非线性分子 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Translation (x, y, and z) | Translation (, , and ) | 翻译(,,和) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Rotation (x, y, and z) | Rotation (, , and ) | 旋转(、和) | 0 | 0 | 0 | 2 | 2 | 2 | 3 | 3 | 3 |
| Total (disregarding Vibration at room temperatures) | Total (disregarding Vibration at room temperatures) | 总计(不包括室温下的振动) | 3 | 3 | 3 | 5 | 5 | 5 | 6 | 6 | 6 |
| Vibration | Vibration | 震动 | 0 | 0 | 0 | 3N − 5 | 3N − 6 | ||||
| Total (including Vibration) | Total (including Vibration) | 总数(包括振动) | 3 | 3 | 3 | 3N | 3N | 3N | 3N | 3N | 3N |
|}
Independent degrees of freedom
The set of degrees of freedom X1, ... , XN of a system is independent if the energy associated with the set can be written in the following form:
The set of degrees of freedom of a system is independent if the energy associated with the set can be written in the following form:
系统的自由度集合是独立的,如果与该自由度集合相关的能量可以写成下列形式:
- [math]\displaystyle{ E = \sum_{i=1}^N E_i(X_i), }[/math]
[math]\displaystyle{ E = \sum_{i=1}^N E_i(X_i), }[/math]
[数学] ,[数学]
where Ei is a function of the sole variable Xi.
where is a function of the sole variable .
这里是唯一变量的函数。
example: if X1 and X2 are two degrees of freedom, and E is the associated energy:
example: if and are two degrees of freedom, and is the associated energy:
例如: 如果和是两个自由度,关联的能量是:
- If [math]\displaystyle{ E = X_1^4 + X_2^4 }[/math], then the two degrees of freedom are independent.
- If [math]\displaystyle{ E = X_1^4 + X_2^4 }[/math], then the two degrees of freedom are independent.
- 如果 < math > e = x _ 1 ^ 4 + x _ 2 ^ 4 </math > ,那么这两个自由度是独立的。
- If [math]\displaystyle{ E = X_1^4 + X_1 X_2 + X_2^4 }[/math], then the two degrees of freedom are not independent. The term involving the product of X1 and X2 is a coupling term that describes an interaction between the two degrees of freedom.
- If [math]\displaystyle{ E = X_1^4 + X_1 X_2 + X_2^4 }[/math], then the two degrees of freedom are not independent. The term involving the product of and is a coupling term that describes an interaction between the two degrees of freedom.
- 如果 < math > e = x 1 ^ 4 + x 1 x 2 + x 2 ^ 4 </math > ,那么这两个自由度不是独立的。包含和乘积的术语是描述两个自由度之间相互作用的耦合术语。
For i from 1 to N, the value of the ith degree of freedom Xi is distributed according to the Boltzmann distribution. Its probability density function is the following:
For from 1 to , the value of the th degree of freedom is distributed according to the Boltzmann distribution. Its probability density function is the following:
对于从1到1,第四自由度的值是按照美国波兹曼分布协会的标准分配的。它的概率密度函数如下:
- [math]\displaystyle{ p_i(X_i) = \frac{e^{-\frac{E_i}{k_B T}}}{\int dX_i \, e^{-\frac{E_i}{k_B T}}} }[/math],
[math]\displaystyle{ p_i(X_i) = \frac{e^{-\frac{E_i}{k_B T}}}{\int dX_i \, e^{-\frac{E_i}{k_B T}}} }[/math],
[数学][数学]
In this section, and throughout the article the brackets [math]\displaystyle{ \langle \rangle }[/math] denote the mean of the quantity they enclose.
In this section, and throughout the article the brackets [math]\displaystyle{ \langle \rangle }[/math] denote the mean of the quantity they enclose.
在本节中,整篇文章中括号 < math > langle rangle </math > 表示它们所包含的量的平均值。
The internal energy of the system is the sum of the average energies associated with each of the degrees of freedom:
The internal energy of the system is the sum of the average energies associated with each of the degrees of freedom:
系统的内能是与每个自由度有关的平均能量之和:
- [math]\displaystyle{ \langle E \rangle = \sum_{i=1}^N \langle E_i \rangle. }[/math]
[math]\displaystyle{ \langle E \rangle = \sum_{i=1}^N \langle E_i \rangle. }[/math]
数学,数学,数学
Quadratic degrees of freedom
A degree of freedom Xi is quadratic if the energy terms associated with this degree of freedom can be written as
A degree of freedom is quadratic if the energy terms associated with this degree of freedom can be written as
如果与这个自由度相关的能量项可以写成二次方程,则该自由度为二次方程
- [math]\displaystyle{ E = \alpha_i\,\,X_i^2 + \beta_i \,\, X_i Y }[/math],
[math]\displaystyle{ E = \alpha_i\,\,X_i^2 + \beta_i \,\, X_i Y }[/math],
数学 e = alpha _ i,,x _ i ^ 2 + beta _ i,,x _ i y,
where Y is a linear combination of other quadratic degrees of freedom.
where is a linear combination of other quadratic degrees of freedom.
其他二次自由度的线性组合。
example: if X1 and X2 are two degrees of freedom, and E is the associated energy:
example: if and are two degrees of freedom, and is the associated energy:
例如: 如果和是两个自由度,关联的能量是:
- If [math]\displaystyle{ E = X_1^4 + X_1^3 X_2 + X_2^4 }[/math], then the two degrees of freedom are not independent and non-quadratic.
- If [math]\displaystyle{ E = X_1^4 + X_1^3 X_2 + X_2^4 }[/math], then the two degrees of freedom are not independent and non-quadratic.
- 如果 < math > e = x _ 1 ^ 4 + x _ 1 ^ 3 x _ 2 + x _ 2 ^ 4 </math > ,那么这两个自由度不是独立的和非二次的。
- If [math]\displaystyle{ E = X_1^4 + X_2^4 }[/math], then the two degrees of freedom are independent and non-quadratic.
- If [math]\displaystyle{ E = X_1^4 + X_2^4 }[/math], then the two degrees of freedom are independent and non-quadratic.
- 如果 < math > e = x _ 1 ^ 4 + x _ 2 ^ 4 </math > ,那么这两个自由度是独立的和非二次的。
- If [math]\displaystyle{ E = X_1^2 + X_1 X_2 + 2X_2^2 }[/math], then the two degrees of freedom are not independent but are quadratic.
- If [math]\displaystyle{ E = X_1^2 + X_1 X_2 + 2X_2^2 }[/math], then the two degrees of freedom are not independent but are quadratic.
- 如果 < math > e = x 1 ^ 2 + x 1 x 2 + 2 x 2 ^ 2 </math > ,那么这两个自由度不是独立的,而是二次的。
- If [math]\displaystyle{ E = X_1^2 + 2X_2^2 }[/math], then the two degrees of freedom are independent and quadratic.
- If [math]\displaystyle{ E = X_1^2 + 2X_2^2 }[/math], then the two degrees of freedom are independent and quadratic.
- 如果 < math > e = x _ 1 ^ 2 + 2 x _ 2 ^ 2 </math > ,那么这两个自由度是独立的和二次的。
For example, in Newtonian mechanics, the dynamics of a system of quadratic degrees of freedom are controlled by a set of homogeneous linear differential equations with constant coefficients.
For example, in Newtonian mechanics, the dynamics of a system of quadratic degrees of freedom are controlled by a set of homogeneous linear differential equations with constant coefficients.
例如,在牛顿运动定律,二次自由度系统的动力学是由一组常系数齐次线性微分方程组控制的。
Quadratic and independent degree of freedom
X1, ... , XN are quadratic and independent degrees of freedom if the energy associated with a microstate of the system they represent can be written as:
are quadratic and independent degrees of freedom if the energy associated with a microstate of the system they represent can be written as:
是二次自由度和独立自由度,如果与它们所代表的系统的微观状态相关的能量可以写成:
- [math]\displaystyle{ E = \sum_{i=1}^N \alpha_i X_i^2 }[/math]
[math]\displaystyle{ E = \sum_{i=1}^N \alpha_i X_i^2 }[/math]
< math > e = sum { i = 1} ^ n alpha _ i x _ i ^ 2
Equipartition theorem
In the classical limit of statistical mechanics, at thermodynamic equilibrium, the internal energy of a system of N quadratic and independent degrees of freedom is:
In the classical limit of statistical mechanics, at thermodynamic equilibrium, the internal energy of a system of quadratic and independent degrees of freedom is:
在统计力学的经典极限下,在热力学平衡,一个二次独立自由度系统的内能是:
- [math]\displaystyle{ U = \langle E \rangle = N\,\frac{k_B T}{2} }[/math]
[math]\displaystyle{ U = \langle E \rangle = N\,\frac{k_B T}{2} }[/math]
[数学] u = langle e rangle = n,frac { k _ b t }{2}[数学]
Here, the mean energy associated with a degree of freedom is:
Here, the mean energy associated with a degree of freedom is:
在这里,与一定自由度相关的平均能是:
- [math]\displaystyle{ \langle E_i \rangle = \int dX_i\,\,\alpha_i X_i^2\,\, p_i(X_i) = \frac{\int dX_i\,\,\alpha_i X_i^2\,\, e^{-\frac{\alpha_i X_i^2}{k_B T}}}{\int dX_i\,\, e^{-\frac{\alpha_i X_i^2}{k_B T}}} }[/math]
[math]\displaystyle{ \langle E_i \rangle = \int dX_i\,\,\alpha_i X_i^2\,\, p_i(X_i) = \frac{\int dX_i\,\,\alpha_i X_i^2\,\, e^{-\frac{\alpha_i X_i^2}{k_B T}}}{\int dX_i\,\, e^{-\frac{\alpha_i X_i^2}{k_B T}}} }[/math]
2,,p _ i (xi) = frac { int dX _ i,,alpha _ i x _ i ^ 2,,e ^ {-frac { alpha _ i x _ i ^ 2,,e ^ {-frac { i x _ i ^ 2}}{ k _ b t }}{ int dX _ i,e ^ {-frac { alpha _ i x _ i ^ 2}}{ k _ b _ i,e ^ {-frac { i x _ i ^ 2}{ k _ b _ t }} </math >
- [math]\displaystyle{ \langle E_i \rangle = \frac{k_B T}{2}\frac{\int dx\,\,x^2\,\, e^{-\frac{x^2}{2}}}{\int dx\,\, e^{-\frac{x^2}{2}}} = \frac{k_B T}{2} }[/math]
[math]\displaystyle{ \langle E_i \rangle = \frac{k_B T}{2}\frac{\int dx\,\,x^2\,\, e^{-\frac{x^2}{2}}}{\int dx\,\, e^{-\frac{x^2}{2}}} = \frac{k_B T}{2} }[/math]
{2} frac { int dx,,x ^ 2,,e ^ {-frac { x ^ 2}{2}}}{ int dx,,e ^ {-frac { x ^ 2}{2}}}{ e ^ {-frac { x ^ 2}{2}}}}} = frac { k _ b }{2} </math >
Since the degrees of freedom are independent, the internal energy of the system is equal to the sum of the mean energy associated with each degree of freedom, which demonstrates the result.
Since the degrees of freedom are independent, the internal energy of the system is equal to the sum of the mean energy associated with each degree of freedom, which demonstrates the result.
由于自由度是独立的,系统的内能等于与每个自由度相关的平均能量之和,从而证明了这一结果。
Generalizations
The description of a system's state as a point in its phase space, although mathematically convenient, is thought to be fundamentally inaccurate. In quantum mechanics, the motion degrees of freedom are superseded with the concept of wave function, and operators which correspond to other degrees of freedom have discrete spectra. For example, intrinsic angular momentum operator (which corresponds to the rotational freedom) for an electron or photon has only two eigenvalues. This discreteness becomes apparent when action has an order of magnitude of the Planck constant, and individual degrees of freedom can be distinguished.
The description of a system's state as a point in its phase space, although mathematically convenient, is thought to be fundamentally inaccurate. In quantum mechanics, the motion degrees of freedom are superseded with the concept of wave function, and operators which correspond to other degrees of freedom have discrete spectra. For example, intrinsic angular momentum operator (which corresponds to the rotational freedom) for an electron or photon has only two eigenvalues. This discreteness becomes apparent when action has an order of magnitude of the Planck constant, and individual degrees of freedom can be distinguished.
将一个系统的状态描述为其相空间中的一个点,虽然在数学上很方便,但被认为是根本不准确的。在量子力学中,运动自由度被波函数的概念所取代,相应于其他自由度的运算符具有离散谱。例如,一个电子或光子的内禀角动量算符(相当于转动自由度)只有两个本征值。当作用力的数量级为普朗克常数时,这种离散性变得显而易见,个体自由度也可以区分。
References
- ↑ 1.0 1.1 Reif, F. (2009). Fundamentals of Statistical and Thermal Physics. Long Grove, IL: Waveland Press, Inc.. p. 51. ISBN 1-57766-612-7.
- ↑ Waldmann, Thomas; Klein, Jens; Hoster, Harry E.; Behm, R. Jürgen (2013). "Stabilization of Large Adsorbates by Rotational Entropy: A Time-Resolved Variable-Temperature STM Study". ChemPhysChem. 14 (1): 162–9. doi:10.1002/cphc.201200531. PMID 23047526.
Category:Concepts in physics
分类: 物理概念
Category:Dimension
类别: 维度
This page was moved from wikipedia:en:Degrees of freedom (physics and chemistry). Its edit history can be viewed at 自由度/edithistory