生物地球化学循环
本词条由张自在初步翻译。
模板:Biogeochemical cycle sidebar
A biogeochemical cycle is the pathway by which a chemical substance cycles (is turned over or moves through) the biotic and the abiotic compartments of Earth. The biotic compartment is the biosphere and the abiotic compartments are the atmosphere, hydrosphere and lithosphere. There are biogeochemical cycles for chemical elements, such as for calcium, carbon, hydrogen, mercury, nitrogen, oxygen, phosphorus, selenium, iron and sulfur, as well as molecular cycles, such as for water and silica. There are also macroscopic cycles, such as the rock cycle, and human-induced cycles for synthetic compounds such as polychlorinated biphenyls (PCBs). In some cycles there are reservoirs where a substance can remain or be sequestered for a long period of time.
A biogeochemical cycle is the pathway by which a chemical substance cycles (is turned over or moves through) the biotic and the abiotic compartments of Earth. The biotic compartment is the biosphere and the abiotic compartments are the atmosphere, hydrosphere and lithosphere. There are biogeochemical cycles for chemical elements, such as for calcium, carbon, hydrogen, mercury, nitrogen, oxygen, phosphorus, selenium, iron and sulfur, as well as molecular cycles, such as for water and silica. There are also macroscopic cycles, such as the rock cycle, and human-induced cycles for synthetic compounds such as polychlorinated biphenyls (PCBs). In some cycles there are reservoirs where a substance can remain or be sequestered for a long period of time.
生物地球化学循环是化学物质在地球的生物隔室和非生物隔室中进行循环(被转化或穿过)的过程。生物隔室指生物圈,非生物隔室指大气圈、水圈和岩石圈。其包括化学元素的生物地球化学循环,如钙、碳、氢、汞、氮、氧、磷、硒、铁和硫,以及分子循环,如水和硅。同时也包括宏观循环,如岩石的循环,以及人为诱导的合成化合物的循环,如多氯联苯(PCBs)的循环。某些循环中存在有储库,储库能使一种物质得以长时间保留或被隔离。
Overview
thumb|upright=1.2|
1.2 |
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.[1]
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.Biogeochemical Cycles , OpenStax, 9 May 2019. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License .
能量在生态系统中定向流动,以阳光(或化能自养生物的无机分子)的形式进入,并在营养级之间的众多转移过程中以热量的形式离开。然而,组成生物体的物质是被保存和循环利用的。与有机分子相关的六种最常见元素——碳、氮、氢、氧、磷和硫——以各种化学形式存在,并可能长期存在于大气、陆地、水体或者地表以下。地质过程,如风化、侵蚀、排水和大陆板块的俯冲,都在这种物质循环中发挥作用。由于地质学和化学在对于该过程的研究中起主要作用,无机物在生物体及其环境之间的循环便被称为生物地球化学循环。
The six aforementioned elements are used by organisms in a variety of ways. Hydrogen and oxygen are found in water and organic molecules, both of which are essential to life. Carbon is found in all organic molecules, whereas nitrogen is an important component of nucleic acids and proteins. Phosphorus is used to make nucleic acids and the phospholipids that comprise biological membranes. Sulfur is critical to the three-dimensional shape of proteins. The cycling of these elements is interconnected. For example, the movement of water is critical for leaching sulfur and phosphorus into rivers which can then flow into oceans. Minerals cycle through the biosphere between the biotic and abiotic components and from one organism to another.[2]
The six aforementioned elements are used by organisms in a variety of ways. Hydrogen and oxygen are found in water and organic molecules, both of which are essential to life. Carbon is found in all organic molecules, whereas nitrogen is an important component of nucleic acids and proteins. Phosphorus is used to make nucleic acids and the phospholipids that comprise biological membranes. Sulfur is critical to the three-dimensional shape of proteins. The cycling of these elements is interconnected. For example, the movement of water is critical for leaching sulfur and phosphorus into rivers which can then flow into oceans. Minerals cycle through the biosphere between the biotic and abiotic components and from one organism to another.Fisher M. R. (Ed.) (2019) Environmental Biology, 3.2 Biogeochemical Cycles , OpenStax. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License .
上述的六种元素以多种方式被生物体利用。氢和氧存在于水和有机分子中,这两种分子对于生命都是必不可少的。所有的有机分子都含有碳,而氮是核酸和蛋白质的重要成分。磷被用来制造核酸和构成生物膜的磷脂。硫对于蛋白质的三维形态至关重要。这些元素的循环是相互关联的。例如,水的流动对于硫和磷渗入河流并流入海洋是至关重要的。矿物质在生物和非生物成分间循环,并从一个生物体转移到另一个,从而在生物圈中进行循环。
Ecological systems (ecosystems) have many biogeochemical cycles operating as a part of the system, for example, the water cycle, the carbon cycle, the nitrogen cycle, etc. All chemical elements occurring in organisms are part of biogeochemical cycles. In addition to being a part of living organisms, these chemical elements also cycle through abiotic factors of ecosystems such as water (hydrosphere), land (lithosphere), and/or the air (atmosphere).[3]
Ecological systems (ecosystems) have many biogeochemical cycles operating as a part of the system, for example, the water cycle, the carbon cycle, the nitrogen cycle, etc. All chemical elements occurring in organisms are part of biogeochemical cycles. In addition to being a part of living organisms, these chemical elements also cycle through abiotic factors of ecosystems such as water (hydrosphere), land (lithosphere), and/or the air (atmosphere).
在生态系统(ecosystems)中有很多生物地球化学循环过程作为系统的一部分运作,如水循环、碳循环、氮循环等等。生物体中所有的化学元素都是生物地球化学循环的一部分。除了作为生物体的一部分,这些化学元素还在生态系统的非生物因子中循环,如水(水圈),陆地(岩石圈)和/或空气(大气圈)。
The living factors of the planet can be referred to collectively as the biosphere. All the nutrients—such as carbon, nitrogen, oxygen, phosphorus, and sulfur—used in ecosystems by living organisms are a part of a closed system; therefore, these chemicals are recycled instead of being lost and replenished constantly such as in an open system.[3]
The living factors of the planet can be referred to collectively as the biosphere. All the nutrients—such as carbon, nitrogen, oxygen, phosphorus, and sulfur—used in ecosystems by living organisms are a part of a closed system; therefore, these chemicals are recycled instead of being lost and replenished constantly such as in an open system.
地球上的生命因素可以统称为生物圈。生态系统中生物体使用的所有营养物质,如碳、氮、氧、磷和硫,都是封闭系统中的一部分。因此,这些化学物质会被循环利用,而不是像在开放系统中那样不断丢失和补充。
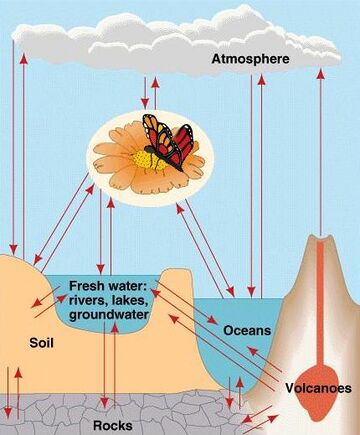
The diagram on the right shows a generalised biogeochemical cycle. The major parts of the biosphere are connected by the flow of chemical elements and compounds. In many of these cycles, the biota plays an important role. Matter from the Earth's interior is released by volcanoes. The atmosphere exchanges some compounds and elements rapidly with the biota and oceans. Exchanges of materials between rocks, soils, and the oceans are generally slower by comparison.[4]
The diagram on the right shows a generalised biogeochemical cycle. The major parts of the biosphere are connected by the flow of chemical elements and compounds. In many of these cycles, the biota plays an important role. Matter from the Earth's interior is released by volcanoes. The atmosphere exchanges some compounds and elements rapidly with the biota and oceans. Exchanges of materials between rocks, soils, and the oceans are generally slower by comparison.Moses, M. (2012) Biogeochemical cycles . Encyclopedia of Earth.
右图展示了一个广义的生物地球化学循环。生物圈的主要部分通过化学元素和化合物的流动相连接。在众多这样的循环中,生物群起了重要的作用。地球内部的物质通过火山喷发释放出来。大气与生物群和海洋之间进行化合物和元素的快速交换。相比之下,岩石、土壤和海洋的物质交换则通常缓慢一些。
The flow of energy in an ecosystem is an open system; the sun constantly gives the planet energy in the form of light while it is eventually used and lost in the form of heat throughout the trophic levels of a food web. Carbon is used to make carbohydrates, fats, and proteins, the major sources of food energy. These compounds are oxidized to release carbon dioxide, which can be captured by plants to make organic compounds. The chemical reaction is powered by the light energy of the sun.
The flow of energy in an ecosystem is an open system; the sun constantly gives the planet energy in the form of light while it is eventually used and lost in the form of heat throughout the trophic levels of a food web. Carbon is used to make carbohydrates, fats, and proteins, the major sources of food energy. These compounds are oxidized to release carbon dioxide, which can be captured by plants to make organic compounds. The chemical reaction is powered by the light energy of the sun.
生态系统中的能量流动是一个开放系统。太阳不断以光能的形式给予地球能量,而这些能量最终以热量的形式在食物网的各营养级中被使用和散失。碳被用来制造碳水化合物、脂肪和蛋白质,它们是食物能量的主要来源。这些化合物被氧化,释放出二氧化碳,这些二氧化碳可以被植物捕获,制造出有机化合物。这种化学反应是由太阳的光能驱动的。
Sunlight is required to combine carbon with hydrogen and oxygen into an energy source, but ecosystems in the deep sea, where no sunlight can penetrate, obtain energy from sulfur. Hydrogen sulfide near hydrothermal vents can be utilized by organisms such as the giant tube worm. In the sulfur cycle, sulfur can be forever recycled as a source of energy. Energy can be released through the oxidation and reduction of sulfur compounds (e.g., oxidizing elemental sulfur to sulfite and then to sulfate).
Sunlight is required to combine carbon with hydrogen and oxygen into an energy source, but ecosystems in the deep sea, where no sunlight can penetrate, obtain energy from sulfur. Hydrogen sulfide near hydrothermal vents can be utilized by organisms such as the giant tube worm. In the sulfur cycle, sulfur can be forever recycled as a source of energy. Energy can be released through the oxidation and reduction of sulfur compounds (e.g., oxidizing elemental sulfur to sulfite and then to sulfate).
将碳与氢和氧结合成能源需要太阳光,但对于阳光无法达及的深海生态系统而言,其从硫中获取能量。热液喷口附近的硫化氢可以被巨型管虫等生物体利用。在硫循环中,硫可以作为能量源被永续循环利用。能量可以通过硫化物的氧化和还原来释放(例如硫被氧化为亚硫酸盐,再被氧化为硫酸盐)。
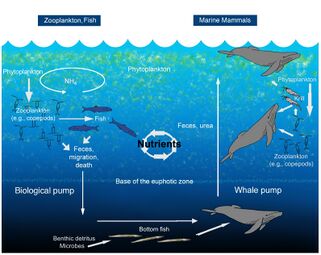
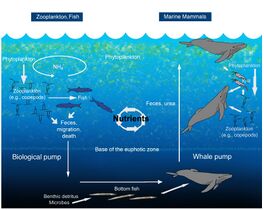
The oceanic whale pump showing how whales cycle nutrients through the ocean water column
- Global carbon cycle.webp
The implications of shifts in the global carbon cycle due to human activity are concerning scientists.[5]
File:BIOGEOCHEMICAL CYCLING OF ELEMENTS.svg|
File:WhalePump.jpg|The oceanic whale pump showing how whales cycle nutrients through the ocean water column
File:Global carbon cycle.webp|The implications of shifts in the global carbon cycle due to human activity are concerning scientists.Avelar, S., van der Voort, T.S. and Eglinton, T.I. (2017) "Relevance of carbon stocks of marine sediments for national greenhouse gas inventories of maritime nations". Carbon balance and management, 12(1): 10.. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License .
文件: 元素的生物地球化学循环.svg | 文件: whalepp.jpg | 海洋中的“鲸泵”显示鲸鱼如何使得养分在大洋水柱中循环: 全球碳循环.webp | 人类活动引起的全球碳循环变化造成的影响令科学家们担忧 | Avelar,s. ,van der Voort,t.s。还有艾格林顿,t.i。(2017年)”海洋沉积物碳储存与海洋国家温室气体清单的相关性”。碳平衡与管理,12(1) : 10。.50px 材料复制自这个来源,可以在知识共享署名4.0国际许可证下获得。
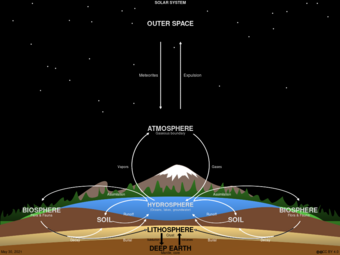
Although the Earth constantly receives energy from the sun, its chemical composition is essentially fixed, as the additional matter is only occasionally added by meteorites. Because this chemical composition is not replenished like energy, all processes that depend on these chemicals must be recycled. These cycles include both the living biosphere and the nonliving lithosphere, atmosphere, and hydrosphere.
Although the Earth constantly receives energy from the sun, its chemical composition is essentially fixed, as the additional matter is only occasionally added by meteorites. Because this chemical composition is not replenished like energy, all processes that depend on these chemicals must be recycled. These cycles include both the living biosphere and the nonliving lithosphere, atmosphere, and hydrosphere.
虽然地球源源不断地从太阳获取能量,但其化学成分基本上是固定的,额外的元素只是偶尔由陨石添加。因为这些化学成分不像能量那样能被补充,所有依赖于这些化学物质的过程都必须能被循环利用。这些循环包括有生命的生物圈和无生命的岩石圈、大气圈和水圈。
Biogeochemical cycles can be contrasted with geochemical cycles. The latter deals only with crustal and subcrustal reservoirs even though some process from both overlap.
Biogeochemical cycles can be contrasted with geochemical cycles. The latter deals only with crustal and subcrustal reservoirs even though some process from both overlap.
生物地球化学循环可以与地球化学循环形成对比。尽管两者的部分过程是重叠的,地球化学循环仅涉及地壳和地下储库。
Reservoirs
The chemicals are sometimes held for long periods of time in one place. This place is called a reservoir, which, for example, includes such things as coal deposits that are storing carbon for a long period of time.[6] When chemicals are held for only short periods of time, they are being held in exchange pools. Examples of exchange pools include plants and animals.[6]
The chemicals are sometimes held for long periods of time in one place. This place is called a reservoir, which, for example, includes such things as coal deposits that are storing carbon for a long period of time. When chemicals are held for only short periods of time, they are being held in exchange pools. Examples of exchange pools include plants and animals.
化学物质有时会在一个地方保存很长时间。这种地方被称作储库,包括诸如能长期储存碳的煤炭沉积。当化学物质只保留很短一段时间时,它们即被存放在交换池中。交换池的例子包括植物和动物。
Plants and animals utilize carbon to produce carbohydrates, fats, and proteins, which can then be used to build their internal structures or to obtain energy. Plants and animals temporarily use carbon in their systems and then release it back into the air or surrounding medium. Generally, reservoirs are abiotic factors whereas exchange pools are biotic factors. Carbon is held for a relatively short time in plants and animals in comparison to coal deposits. The amount of time that a chemical is held in one place is called its residence time or turnover time (also called the renewal time or exit age).[6]
Plants and animals utilize carbon to produce carbohydrates, fats, and proteins, which can then be used to build their internal structures or to obtain energy. Plants and animals temporarily use carbon in their systems and then release it back into the air or surrounding medium. Generally, reservoirs are abiotic factors whereas exchange pools are biotic factors. Carbon is held for a relatively short time in plants and animals in comparison to coal deposits. The amount of time that a chemical is held in one place is called its residence time or turnover time (also called the renewal time or exit age).
动植物利用碳来生产碳水化合物、脂肪和蛋白质,这些可之后被用于构建它们的内部结构或获取能量。植物和动物短暂地使用它们系统中的碳,随后将其释放到空气或周围的介质中。一般来说,储库是非生物因子,交换池是生物因子。与煤炭层相比,碳在动植物体内只保存相对较短的一段时间。一种化学物质在一个地方保留的时间被称为其停留时间或周转时间(也称为更新时间或退出时间)。
Box models
thumb|upright=1|right|
= = 箱模型 = = 拇指 | 竖直 = 1 | 右 |
Box models are widely used to model biogeochemical systems.[7][8] Box models are simplified versions of complex systems, reducing them to boxes (or storage reservoirs) for chemical materials, linked by material fluxes (flows). Simple box models have a small number of boxes with properties, such as volume, that do not change with time. The boxes are assumed to behave as if they were mixed homogeneously.[8] These models are often used to derive analytical formulas describing the dynamics and steady-state abundance of the chemical species involved.
Box models are widely used to model biogeochemical systems.Bianchi, Thomas (2007) Biogeochemistry of Estuaries page 9, Oxford University Press. . Box models are simplified versions of complex systems, reducing them to boxes (or storage reservoirs) for chemical materials, linked by material fluxes (flows). Simple box models have a small number of boxes with properties, such as volume, that do not change with time. The boxes are assumed to behave as if they were mixed homogeneously. These models are often used to derive analytical formulas describing the dynamics and steady-state abundance of the chemical species involved.
箱模型被广泛用于生物地球化学系统建模。箱模型是复杂系统的简化版本,将其简化为存放化学物质的箱(或储库),并由物质通量(流)进行连接。简单的箱模型含有少量属性不随时间变化的箱室,例如体积。这些箱室的行为被假定为是均匀混合的。这些模型经常被用于推导出解析公式以描述所涉及的化学物质的动力学和稳态丰度。
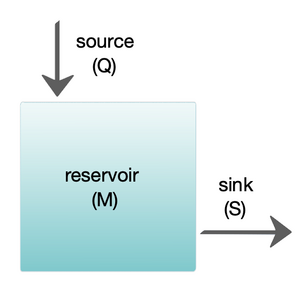
The diagram at the right shows a basic one-box model. The reservoir contains the amount of material M under consideration, as defined by chemical, physical or biological properties. The source Q is the flux of material into the reservoir, and the sink S is the flux of material out of the reservoir. The budget is the check and balance of the sources and sinks affecting material turnover in a reservoir. The reservoir is in a steady state if Q = S, that is, if the sources balance the sinks and there is no change over time.[8]
The diagram at the right shows a basic one-box model. The reservoir contains the amount of material M under consideration, as defined by chemical, physical or biological properties. The source Q is the flux of material into the reservoir, and the sink S is the flux of material out of the reservoir. The budget is the check and balance of the sources and sinks affecting material turnover in a reservoir. The reservoir is in a steady state if Q = S, that is, if the sources balance the sinks and there is no change over time.
右图展示了一个基本的单箱模型。储库包含了所考虑的物质M的量,按照化学、物理或生物属性的定义。源Q是流入储库的物质通量,汇S是流出储库的物质通量。预算是影响储库内物质周转的源和汇的制衡。当Q = S,即源与汇处于平衡状态且不随时间变化时,储库处于稳态。
The residence or turnover time is the average time material spends resident in the reservoir. If the reservoir is in a steady state, this is the same as the time it takes to fill or drain the reservoir. Thus, if τ is the turnover time, then τ = M/S.[8] The equation describing the rate of change of content in a reservoir is
The residence or turnover time is the average time material spends resident in the reservoir. If the reservoir is in a steady state, this is the same as the time it takes to fill or drain the reservoir. Thus, if τ is the turnover time, then τ = M/S. The equation describing the rate of change of content in a reservoir is
停留时间或周转时间是物质在储库中停留的平均时间。如果储库处于稳态,其等于蓄满或排空储库所需的时间。因此,如果τ 是周转时间,那么 τ = M/S。描述储库物质含量变化率的方程为
- [math]\displaystyle{ \frac{dM}{dt} = Q - S = Q - \frac{M}{\tau} }[/math]
- \frac{dM}{dt} = Q - S = Q - \frac{M}{\tau}
- frac { dM }{ dt } = q-s = q-frac { m }{ tau }
When two or more reservoirs are connected, the material can be regarded as cycling between the reservoirs, and there can be predictable patterns to the cyclic flow.[8] More complex multibox models are usually solved using numerical techniques.
When two or more reservoirs are connected, the material can be regarded as cycling between the reservoirs, and there can be predictable patterns to the cyclic flow. More complex multibox models are usually solved using numerical techniques.
当两个或多个储库相连通时,可以认为物质在储库之间循环,且循环流动具有可预测的模式。更复杂的多箱模型通常用数值方法求解。
thumb|upright=2.2|right|
2.2 | right |
The diagram on the left above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the euphotic zone, one for the ocean interior or dark ocean, and one for ocean sediments. In the euphotic zone, net phytoplankton production is about 50 Pg C each year. About 10 Pg is exported to the ocean interior while the other 40 Pg is respired. Organic carbon degradation occurs as particles (marine snow) settle through the ocean interior. Only 2 Pg eventually arrives at the seafloor, while the other 8 Pg is respired in the dark ocean. In sediments, the time scale available for degradation increases by orders of magnitude with the result that 90% of the organic carbon delivered is degraded and only 0.2 Pg C yr−1 is eventually buried and transferred from the biosphere to the geosphere.[9]
The diagram on the left above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the euphotic zone, one for the ocean interior or dark ocean, and one for ocean sediments. In the euphotic zone, net phytoplankton production is about 50 Pg C each year. About 10 Pg is exported to the ocean interior while the other 40 Pg is respired. Organic carbon degradation occurs as particles (marine snow) settle through the ocean interior. Only 2 Pg eventually arrives at the seafloor, while the other 8 Pg is respired in the dark ocean. In sediments, the time scale available for degradation increases by orders of magnitude with the result that 90% of the organic carbon delivered is degraded and only 0.2 Pg C yr−1 is eventually buried and transferred from the biosphere to the geosphere.
左上图显示了海洋碳流动的简化预算。它由三个相互连通的简单箱模型组成,一个是真光层,一个是海洋内部或深海,一个是海洋沉积物。在真光层,浮游植物每年净生产量约为50Pg。大约10Pg被输送到海洋内部,其余40Pg则被呼吸作用消耗。有机碳的降解发生在颗粒(海洋雪)在海洋内部沉降的过程中。只有2Pg的碳最终到达海底,其余的8Pg在深海中被呼吸作用消耗。在沉积物中,可供降解的时间尺度增加了一个数量级,导致90%的有机碳被降解,最终只有0.2PgC yr-1被埋藏并从生物圈转移到地圈。
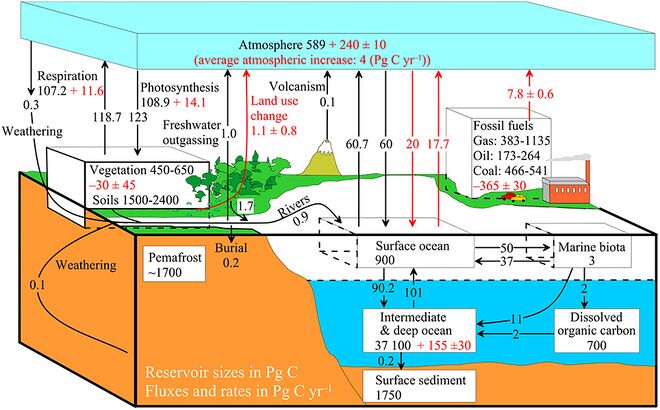
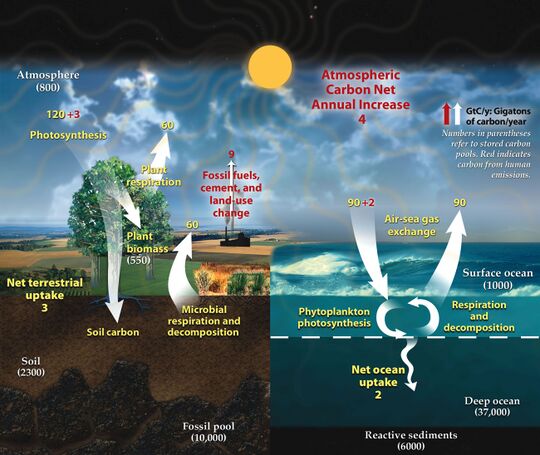
The diagram on the right above shows a more complex model with many interacting boxes. Reservoir masses here represents carbon stocks, measured in Pg C. Carbon exchange fluxes, measured in Pg C yr−1, occur between the atmosphere and its two major sinks, the land and the ocean. The black numbers and arrows indicate the reservoir mass and exchange fluxes estimated for the year 1750, just before the Industrial Revolution. The red arrows (and associated numbers) indicate the annual flux changes due to anthropogenic activities, averaged over the 2000–2009 time period. They represent how the carbon cycle has changed since 1750. Red numbers in the reservoirs represent the cumulative changes in anthropogenic carbon since the start of the Industrial Period, 1750–2011.[10][11][12]
The diagram on the right above shows a more complex model with many interacting boxes. Reservoir masses here represents carbon stocks, measured in Pg C. Carbon exchange fluxes, measured in Pg C yr−1, occur between the atmosphere and its two major sinks, the land and the ocean. The black numbers and arrows indicate the reservoir mass and exchange fluxes estimated for the year 1750, just before the Industrial Revolution. The red arrows (and associated numbers) indicate the annual flux changes due to anthropogenic activities, averaged over the 2000–2009 time period. They represent how the carbon cycle has changed since 1750. Red numbers in the reservoirs represent the cumulative changes in anthropogenic carbon since the start of the Industrial Period, 1750–2011. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License .
右上图显示了一个更复杂的模型,其中包含了许多相互作用的箱室。这里储库的质量代表碳储量,以Pg C为单位。碳交换通量以Pg C yr-1为单位,出现于大气和两个主要的碳汇,陆地和海洋之间。黑色的数字和箭头表示了1750年(工业革命之前)的碳库含量和交换通量的估计值。红色的箭头和对应的数字代表了2000-2009年人类活动导致的碳通量变化的年平均值。它们显示了1750年以来碳循环的变化情况。储库中的红色数字表示了自1750年至2011年,即工业革命开始以来人为碳的累积变化。
Compartments
Compartments
= 隔室 =
Biosphere
Microorganisms drive much of the biogeochemical cycling in the earth system.[13][14]
Microorganisms drive much of the biogeochemical cycling in the earth system. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License .
微生物驱动了地球系统中大部分的生物地球化学循环。
Atmosphere
Hydrosphere
The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and coastal ecosystems comprise a minor fraction of the ocean in terms of surface area, yet have an enormous impact on global biogeochemical cycles carried out by microbial communities, which represent 90% of the ocean's biomass.[15] Work in recent years has largely focused on cycling of carbon and macronutrients such as nitrogen, phosphorus, and silicate: other important elements such as sulfur or trace elements have been less studied, reflecting associated technical and logistical issues.[16] Increasingly, these marine areas, and the taxa that form their ecosystems, are subject to significant anthropogenic pressure, impacting marine life and recycling of energy and nutrients.[17][18][19] A key example is that of cultural eutrophication, where agricultural runoff leads to nitrogen and phosphorus enrichment of coastal ecosystems, greatly increasing productivity resulting in algal blooms, deoxygenation of the water column and seabed, and increased greenhouse gas emissions,[20] with direct local and global impacts on nitrogen and carbon cycles. However, the runoff of organic matter from the mainland to coastal ecosystems is just one of a series of pressing threats stressing microbial communities due to global change. Climate change has also resulted in changes in the cryosphere, as glaciers and permafrost melt, resulting in intensified marine stratification, while shifts of the redox-state in different biomes are rapidly reshaping microbial assemblages at an unprecedented rate.[21][22][23][24][16]
The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and coastal ecosystems comprise a minor fraction of the ocean in terms of surface area, yet have an enormous impact on global biogeochemical cycles carried out by microbial communities, which represent 90% of the ocean's biomass. Work in recent years has largely focused on cycling of carbon and macronutrients such as nitrogen, phosphorus, and silicate: other important elements such as sulfur or trace elements have been less studied, reflecting associated technical and logistical issues. Increasingly, these marine areas, and the taxa that form their ecosystems, are subject to significant anthropogenic pressure, impacting marine life and recycling of energy and nutrients.Galton, D. (1884) 10th Meeting: report of the royal commission on metropolitan sewage . J. Soc. Arts, 33: 290. A key example is that of cultural eutrophication, where agricultural runoff leads to nitrogen and phosphorus enrichment of coastal ecosystems, greatly increasing productivity resulting in algal blooms, deoxygenation of the water column and seabed, and increased greenhouse gas emissions, with direct local and global impacts on nitrogen and carbon cycles. However, the runoff of organic matter from the mainland to coastal ecosystems is just one of a series of pressing threats stressing microbial communities due to global change. Climate change has also resulted in changes in the cryosphere, as glaciers and permafrost melt, resulting in intensified marine stratification, while shifts of the redox-state in different biomes are rapidly reshaping microbial assemblages at an unprecedented rate. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License .
海洋覆盖了地球表面的70%以上,并且具有很强的异质性。海洋生产区和沿海生态系统只占海洋表面积的一小部分,但对微生物群落(占海洋生物量的90%)所进行的全球生物地球化学循环有着巨大的影响。近年来的工作主要集中在碳和常量营养元素(如氮、磷和硅酸盐)的循环上,对其它重要元素(如硫或微量元素)的研究较少,反映的相关的技术和后勤问题。这些海域以及构成其生态系统的分类群正日益受到人类活动的巨大压力,影响着海洋生物以及能量和营养物质的循环。一个关键的例子是人为富营养化的影响,农业生产的径流导致沿海生态系统的氮和磷富集,使生产力大大提高并造成藻类大量繁殖,水体和海床脱氧,温室气体排放增加,直接影响了区域和全球的氮循环和碳循环。然而,有机物从大陆流入沿海生态系统只是全球变化对微生物群落造成的一系列胁迫之一。气候变化还导致了冰冻圈的变化,冰川和永久冻土融化加剧了海洋分层,而不同生物群落中氧化还原状态的变化正以前所未有的速度迅速重塑微生物组合。
Global change is, therefore, affecting key processes including primary productivity, CO2 and N2 fixation, organic matter respiration/remineralization, and the sinking and burial deposition of fixed CO2.[24] In addition to this, oceans are experiencing an acidification process, with a change of ~0.1 pH units between the pre-industrial period and today, affecting carbonate/bicarbonate buffer chemistry. In turn, acidification has been reported to impact planktonic communities, principally through effects on calcifying taxa.[25] There is also evidence for shifts in the production of key intermediary volatile products, some of which have marked greenhouse effects (e.g., N2O and CH4, reviewed by Breitburg in 2018,[22] due to the increase in global temperature, ocean stratification and deoxygenation, driving as much as 25 to 50% of nitrogen loss from the ocean to the atmosphere in the so-called oxygen minimum zones模板:Hsp[26] or anoxic marine zones,[27] driven by microbial processes. Other products, that are typically toxic for the marine nekton, including reduced sulfur species such as H2S, have a negative impact for marine resources like fisheries and coastal aquaculture. While global change has accelerated, there has been a parallel increase in awareness of the complexity of marine ecosystems, and especially the fundamental role of microbes as drivers of ecosystem functioning.[23][16]
Global change is, therefore, affecting key processes including primary productivity, CO2 and N2 fixation, organic matter respiration/remineralization, and the sinking and burial deposition of fixed CO2. In addition to this, oceans are experiencing an acidification process, with a change of ~0.1 pH units between the pre-industrial period and today, affecting carbonate/bicarbonate buffer chemistry. In turn, acidification has been reported to impact planktonic communities, principally through effects on calcifying taxa. There is also evidence for shifts in the production of key intermediary volatile products, some of which have marked greenhouse effects (e.g., N2O and CH4, reviewed by Breitburg in 2018, due to the increase in global temperature, ocean stratification and deoxygenation, driving as much as 25 to 50% of nitrogen loss from the ocean to the atmosphere in the so-called oxygen minimum zones or anoxic marine zones, driven by microbial processes. Other products, that are typically toxic for the marine nekton, including reduced sulfur species such as H2S, have a negative impact for marine resources like fisheries and coastal aquaculture. While global change has accelerated, there has been a parallel increase in awareness of the complexity of marine ecosystems, and especially the fundamental role of microbes as drivers of ecosystem functioning.
因此,全球变化正在影响着关键过程,包括净初级生产力,CO2和N2固定,有机物呼吸/再矿化以及固定CO2的沉积和埋藏。除此之外,海洋正在经历酸化过程,从前工业化时期到如今pH值变化了约0.1个单位,影响了碳酸盐和碳酸氢盐缓冲的化学过程。反过来,酸化主要通过对钙化类群的影响从而影响浮游生物群落。还有证据表明,关键的中间挥发性产物的生产过程发生了变化,其中一些产物具有明显的温室效应(例如N2O和CH4,Breitburg在2018年的综述中所言。由于全球温度升高,海洋分层和脱氧,在所谓的最低含氧区或缺氧海洋区由于微生物的驱动导致大洋中25%-50%的氮损失到大气中)。其它对海洋自游生物有毒的产物,包括诸如H2S等硫的还原产物,对渔业和沿海水产养殖等海洋资源有负面影响。虽然全球变化加速,但人们对海洋生态系统复杂性的认识也在同步提高,尤其是微生物作为生态系统功能驱动因素的基本作用。
Lithosphere
Fast and slow cycles
There are fast and slow biogeochemical cycles. Fast cycle operate in the biosphere and slow cycles operate in rocks. Fast or biological cycles can complete within years, moving substances from atmosphere to biosphere, then back to the atmosphere. Slow or geological cycles can take millions of years to complete, moving substances through the Earth's crust between rocks, soil, ocean and atmosphere.[28]
There are fast and slow biogeochemical cycles. Fast cycle operate in the biosphere and slow cycles operate in rocks. Fast or biological cycles can complete within years, moving substances from atmosphere to biosphere, then back to the atmosphere. Slow or geological cycles can take millions of years to complete, moving substances through the Earth's crust between rocks, soil, ocean and atmosphere.Libes, Susan M. (2015). Blue planet: The role of the oceans in nutrient cycling, maintain the atmosphere system, and modulating climate change In: Routledge Handbook of Ocean Resources and Management, Routledge, pages 89–107. .
生物地球化学循环包括快速和慢速循环。快速循环在生物圈中运行,慢速循环在岩石中运行。快速循环或生物循环可以在数年内完成,将物质从大气转移到生物圈,再转移到大气。慢速或地质循环可能需要数百万年才能完成,使物质穿过岩石、土壤、海洋和大气在地壳中运移。.
As an example, the fast carbon cycle is illustrated in the diagram below on the left. This cycle involves relatively short-term biogeochemical processes between the environment and living organisms in the biosphere. It includes movements of carbon between the atmosphere and terrestrial and marine ecosystems, as well as soils and seafloor sediments. The fast cycle includes annual cycles involving photosynthesis and decadal cycles involving vegetative growth and decomposition. The reactions of the fast carbon cycle to human activities will determine many of the more immediate impacts of climate change.[29][30][31][32]
As an example, the fast carbon cycle is illustrated in the diagram below on the left. This cycle involves relatively short-term biogeochemical processes between the environment and living organisms in the biosphere. It includes movements of carbon between the atmosphere and terrestrial and marine ecosystems, as well as soils and seafloor sediments. The fast cycle includes annual cycles involving photosynthesis and decadal cycles involving vegetative growth and decomposition. The reactions of the fast carbon cycle to human activities will determine many of the more immediate impacts of climate change. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License .
快速的碳循环案例如左下图所示。这一循环包括环境和生物圈中生命体之间相对短期的地球化学过程。它包括碳在大气以及陆地和海洋生态系统、泥土和海底沉积物之间的运移。快速循环包括涉及光合作用的年周期和涉及植被生长和分解的年代周期。快速碳循环对人类活动的响应将决定气候变化许多更直接的影响。
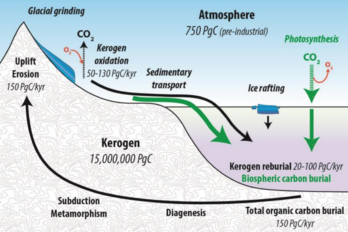
thumb|upright=1.8|left| The fast cycle operates through the biosphere, including exchanges between land, atmosphere, and oceans. The yellow numbers are natural fluxes of carbon in billions of tons (gigatons) per year. Red are human contributions and white are stored carbon.
快速循环在生物圈中运行,包括陆地、大气层和海洋之间的交换。黄色数字是每年数十亿吨碳的自然通量。红色是人类的贡献,白色是储存的碳。
thumb|upright=2.25|right|
2.25
The slow cycle is illustrated in the diagram above on the right. It involves medium to long-term geochemical processes belonging to the rock cycle. The exchange between the ocean and atmosphere can take centuries, and the weathering of rocks can take millions of years. Carbon in the ocean precipitates to the ocean floor where it can form sedimentary rock and be subducted into the earth's mantle. Mountain building processes result in the return of this geologic carbon to the Earth's surface. There the rocks are weathered and carbon is returned to the atmosphere by degassing and to the ocean by rivers. Other geologic carbon returns to the ocean through the hydrothermal emission of calcium ions. In a given year between 10 and 100 million tonnes of carbon moves around this slow cycle. This includes volcanoes returning geologic carbon directly to the atmosphere in the form of carbon dioxide. However, this is less than one percent of the carbon dioxide put into the atmosphere by burning fossil fuels.[28][29]
The slow cycle is illustrated in the diagram above on the right. It involves medium to long-term geochemical processes belonging to the rock cycle. The exchange between the ocean and atmosphere can take centuries, and the weathering of rocks can take millions of years. Carbon in the ocean precipitates to the ocean floor where it can form sedimentary rock and be subducted into the earth's mantle. Mountain building processes result in the return of this geologic carbon to the Earth's surface. There the rocks are weathered and carbon is returned to the atmosphere by degassing and to the ocean by rivers. Other geologic carbon returns to the ocean through the hydrothermal emission of calcium ions. In a given year between 10 and 100 million tonnes of carbon moves around this slow cycle. This includes volcanoes returning geologic carbon directly to the atmosphere in the form of carbon dioxide. However, this is less than one percent of the carbon dioxide put into the atmosphere by burning fossil fuels.
缓慢的循环在上面右边的图表中显示出来。它涉及中长期的地球化学过程,属于岩石旋回。海洋和大气之间的交换可能需要几个世纪,岩石的风化可能需要几百万年。海洋中的碳沉淀到海底,在那里它可以形成沉积岩并潜入地幔。造山过程导致这种地质碳返回到地球表面。在那里,岩石被风化,碳通过排气返回大气层,通过河流流入海洋。其他地质碳通过钙离子的水热释放返回海洋。在给定的一年中,1000万到1亿吨的碳在这个缓慢的循环周期中移动。这包括火山以二氧化碳的形式将地质碳直接返回大气层。然而,这还不到燃烧化石燃料排放到大气中的二氧化碳的百分之一。
Deep cycles
The terrestrial subsurface is the largest reservoir of carbon on earth, containing 14–135 Pg of carbon模板:Hsp[34] and 2–19% of all biomass.[35] Microorganisms drive organic and inorganic compound transformations in this environment and thereby control biogeochemical cycles. Current knowledge of the microbial ecology of the subsurface is primarily based on 16S ribosomal RNA (rRNA) gene sequences. Recent estimates show that <8% of 16S rRNA sequences in public databases derive from subsurface organisms模板:Hsp[36] and only a small fraction of those are represented by genomes or isolates. Thus, there is remarkably little reliable information about microbial metabolism in the subsurface. Further, little is known about how organisms in subsurface ecosystems are metabolically interconnected. Some cultivation-based studies of syntrophic consortia模板:Hsp[37][38][39] and small-scale metagenomic analyses of natural communities模板:Hsp[40][41][42] suggest that organisms are linked via metabolic handoffs: the transfer of redox reaction products of one organism to another. However, no complex environments have been dissected completely enough to resolve the metabolic interaction networks that underpin them. This restricts the ability of biogeochemical models to capture key aspects of the carbon and other nutrient cycles.[43] New approaches such as genome-resolved metagenomics, an approach that can yield a comprehensive set of draft and even complete genomes for organisms without the requirement for laboratory isolation模板:Hsp[40][44][45] have the potential to provide this critical level of understanding of biogeochemical processes.[46]
The terrestrial subsurface is the largest reservoir of carbon on earth, containing 14–135 Pg of carbon and 2–19% of all biomass. Microorganisms drive organic and inorganic compound transformations in this environment and thereby control biogeochemical cycles. Current knowledge of the microbial ecology of the subsurface is primarily based on 16S ribosomal RNA (rRNA) gene sequences. Recent estimates show that <8% of 16S rRNA sequences in public databases derive from subsurface organisms and only a small fraction of those are represented by genomes or isolates. Thus, there is remarkably little reliable information about microbial metabolism in the subsurface. Further, little is known about how organisms in subsurface ecosystems are metabolically interconnected. Some cultivation-based studies of syntrophic consortia and small-scale metagenomic analyses of natural communities suggest that organisms are linked via metabolic handoffs: the transfer of redox reaction products of one organism to another. However, no complex environments have been dissected completely enough to resolve the metabolic interaction networks that underpin them. This restricts the ability of biogeochemical models to capture key aspects of the carbon and other nutrient cycles. New approaches such as genome-resolved metagenomics, an approach that can yield a comprehensive set of draft and even complete genomes for organisms without the requirement for laboratory isolation have the potential to provide this critical level of understanding of biogeochemical processes. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License .
陆地地下是地球上最大的碳储存库,含有14-135 Pg 的碳,占所有生物量的2-19% 。微生物在这种环境中推动有机和无机化合物的转变,从而控制生物地球化学循环。目前对地下微生物生态学的了解主要是基于16S 核糖体RNA 基因序列。最近的估计显示,公共数据库中的16s rRNA 序列中,少于8% 来自地下生物,其中只有一小部分由基因组或分离物表示。因此,关于地下微生物代谢的可靠信息非常少。此外,关于地下生态系统中的生物体是如何在新陈代谢上相互关联的,我们知之甚少。一些以培养为基础的联合营养研究和对自然群落的小规模宏基因组学分析表明,生物体之间是通过代谢传递联系起来的: 一种生物体的氧化还原反应产物转移到另一种生物体。然而,还没有一个复杂的环境被彻底剖析,足以解析支撑它们的代谢交互网络。这限制了生物地球化学模型捕捉碳和其他养分循环关键方面的能力。基因组分解宏基因组学等新的方法可以为生物体提供一套全面的草图甚至完整的基因组,而不需要实验室隔离,这种方法有可能提供对生物地球化学过程的这一关键水平的理解。50px 材料复制自这个来源,可以在知识共享署名4.0国际许可证下获得。
Some examples
Some of the more well-known biogeochemical cycles are shown below:
Some of the more well-known biogeochemical cycles are shown below:
= = = 一些例子 = = 一些比较著名的生物地球化学循环如下:
File:Carbon cycle-cute diagram.svg|alt=Diagram of the carbon cycle|Carbon cycle
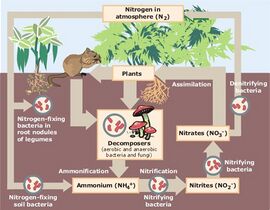
File:Nitrogen_Cycle.jpg|alt=Diagram of the nitrogen cycle|Nitrogen cycle
File:WhalePump.jpg|alt=Diagram of the nutrient cycle|Nutrient cycle
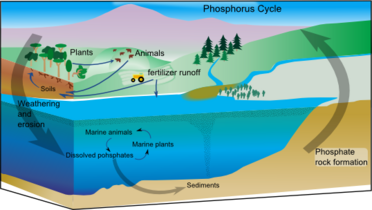
File:Phosphorus cycle.png|alt=Diagram of the phosphorus cycle|Phosphorus cycle
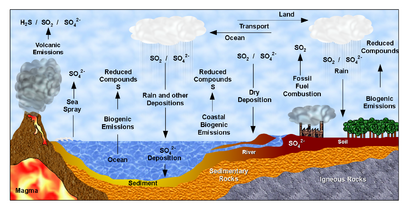
File:Sulfur Cycle (Ciclo do Enxofre).png|alt=Diagram of the sulfur cycle|Sulfur cycle
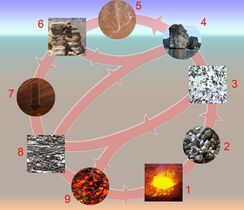
File:Rockcycle.jpg|alt=Diagram of the rock cycle|Rock cycle
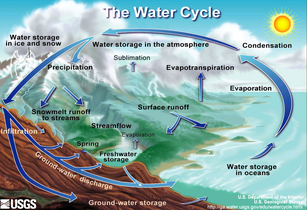
File:Water cycle.png|alt=Diagram of the water cycle|Water cycle
文件: 碳循环-可爱的图解. svg | alt = 碳循环图 | 碳循环文件: 氮循环。氮循环图 | 氮循环文件: whaleump.jpg | alt = 营养循环图 | 营养循环文件: p Cycle.png | alt = 磷循环循环图 | 磷循环循环文件: 硫循环(Ciclo do Enxofre)。硫循环图 | 硫循环图 | 岩石循环图 | 岩石循环图 | 水循环图 | 水循环图
Many biogeochemical cycles are currently being studied for the first time. Climate change and human impacts are drastically changing the speed, intensity, and balance of these relatively unknown cycles, which include:
- the mercury cycle,[47] and
- the human-caused cycle of PCBs.[48]
Chloroplasts conduct photosynthesis in plant cells and other eukaryotic organisms.
Many biogeochemical cycles are currently being studied for the first time. Climate change and human impacts are drastically changing the speed, intensity, and balance of these relatively unknown cycles, which include:
- the mercury cycle, and
- the human-caused cycle of PCBs.
File:Plagiomnium affine laminazellen.jpeg|Chloroplasts conduct photosynthesis in plant cells and other eukaryotic organisms. File:Organic carbon cycle including the flow of kerogen.png|Kerogen cycle File:Coal anthracite.jpg|Coal is a reservoir of carbon
目前许多生物地球化学循环的研究尚属首次。气候变化和人类活动的影响正在极大地改变这些相对未知的循环的速度、强度和平衡,其中包括:
- 汞循环,
- 多氯联苯的人为循环。文件: 寒地走灯藓/叶绿体在植物细胞和其他真核生物中进行光合作用。文件: 有机碳循环包括干酪根的流动
Biogeochemical cycles always involve active equilibrium states: a balance in the cycling of the element between compartments. However, overall balance may involve compartments distributed on a global scale.
Biogeochemical cycles always involve active equilibrium states: a balance in the cycling of the element between compartments. However, overall balance may involve compartments distributed on a global scale.
生物地球化学循环总是涉及活动平衡状态: 元素在区间循环中的平衡。然而,总体平衡可能涉及在全球范围内分布的部门。
As biogeochemical cycles describe the movements of substances on the entire globe, the study of these is inherently multidisciplinary. The carbon cycle may be related to research in ecology and atmospheric sciences.[51] Biochemical dynamics would also be related to the fields of geology and pedology.[52]
As biogeochemical cycles describe the movements of substances on the entire globe, the study of these is inherently multidisciplinary. The carbon cycle may be related to research in ecology and atmospheric sciences. Biochemical dynamics would also be related to the fields of geology and pedology.
由于生物地球化学循环描述了物质在整个地球上的运动,对这些运动的研究本质上是多学科的。碳循环可能与生态学和大气科学研究有关。生物化学动力学也与地质学和土壤学领域有关。
History
See also
- Carbonate–silicate cycle
- Ecological recycling
- Great Acceleration
- Hydrogen cycle
- Marine biogeochemical cycles
- Redox gradient
= =
- 碳酸盐-硅酸盐循环
- 生态循环
- 大加速度
- 氢循环
- 海洋生物地球化学循环
- 氧化还原梯度
References
- ↑ Biogeochemical Cycles -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2021-09-27., OpenStax, 9 May 2019. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2017-10-16..
- ↑ Fisher M. R. (Ed.) (2019) Environmental Biology, 3.2 Biogeochemical Cycles -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2021-09-27., OpenStax. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2017-10-16..
- ↑ 3.0 3.1 "Biogeochemical Cycles". The Environmental Literacy Council. Archived from the original on 30 April 2015. Retrieved 20 November 2017.
- ↑ Moses, M. (2012) Biogeochemical cycles -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2021-11-22.. Encyclopedia of Earth.
- ↑ Avelar, S., van der Voort, T.S. and Eglinton, T.I. (2017) "Relevance of carbon stocks of marine sediments for national greenhouse gas inventories of maritime nations". Carbon balance and management, 12(1): 10.doi:10.1186/s13021-017-0077-x. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2017-10-16..
- ↑ 6.0 6.1 6.2 Baedke, Steve J.; Fichter, Lynn S. "Biogeochemical Cycles: Carbon Cycle". Supplimental Lecture Notes for Geol 398. James Madison University. Archived from the original on 1 December 2017. Retrieved 20 November 2017.
- ↑ Sarmiento, J.L.; Toggweiler, J.R. (1984). "A new model for the role of the oceans in determining atmospheric P CO 2". Nature. 308 (5960): 621–24. Bibcode:1984Natur.308..621S. doi:10.1038/308621a0. S2CID 4312683.
- ↑ 8.0 8.1 8.2 8.3 8.4 Bianchi, Thomas (2007) Biogeochemistry of Estuaries -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2021-09-25. page 9, Oxford University Press. .
- ↑ 引用错误:无效
<ref>标签;未给name属性为Middelburg2019的引用提供文字 - ↑ Sarmiento, Jorge L.; Gruber, Nicolas (2002). "Sinks for Anthropogenic Carbon". Physics Today. 55 (8): 30–36. Bibcode:2002PhT....55h..30S. doi:10.1063/1.1510279.
- ↑ Chhabra, Abha (2013). "Carbon and Other Biogeochemical Cycles". doi:10.13140/2.1.1081.8883.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Kandasamy, Selvaraj; Nagender Nath, Bejugam (2016). "Perspectives on the Terrestrial Organic Matter Transport and Burial along the Land-Deep Sea Continuum: Caveats in Our Understanding of Biogeochemical Processes and Future Needs". Frontiers in Marine Science. 3. doi:10.3389/fmars.2016.00259. S2CID 30408500. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2017-10-16..
- ↑ Falkowski, P. G.; Fenchel, T.; Delong, E. F. (2008). "The Microbial Engines That Drive Earth's Biogeochemical Cycles". Science. 320 (5879): 1034–1039. Bibcode:2008Sci...320.1034F. doi:10.1126/science.1153213. PMID 18497287. S2CID 2844984.
- ↑ Zakem, Emily J.; Polz, Martin F.; Follows, Michael J. (2020). "Redox-informed models of global biogeochemical cycles". Nature Communications. 11 (1): 5680. Bibcode:2020NatCo..11.5680Z. doi:10.1038/s41467-020-19454-w. PMC 7656242. PMID 33173062. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2017-10-16..
- ↑ Alexander, Vera; Miloslavich, Patricia; Yarincik, Kristen (2011). "The Census of Marine Life—evolution of worldwide marine biodiversity research". Marine Biodiversity. 41 (4): 545–554. doi:10.1007/s12526-011-0084-1. S2CID 25888475.
- ↑ 16.0 16.1 16.2 Murillo, Alejandro A.; Molina, Verónica; Salcedo-Castro, Julio; Harrod, Chris (2019). "Editorial: Marine Microbiome and Biogeochemical Cycles in Marine Productive Areas". Frontiers in Marine Science. 6. doi:10.3389/fmars.2019.00657. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2017-10-16..
- ↑ Galton, D. (1884) 10th Meeting: report of the royal commission on metropolitan sewage -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2021-09-24.. J. Soc. Arts, 33: 290.
- ↑ Hasler, Arthur D. (1969). "Cultural Eutrophication is Reversible". BioScience. 19 (5): 425–431. doi:10.2307/1294478. JSTOR 1294478.
- ↑ Jickells, T. D.; Buitenhuis, E.; Altieri, K.; Baker, A. R.; Capone, D.; Duce, R. A.; Dentener, F.; Fennel, K.; Kanakidou, M.; Laroche, J.; Lee, K.; Liss, P.; Middelburg, J. J.; Moore, J. K.; Okin, G.; Oschlies, A.; Sarin, M.; Seitzinger, S.; Sharples, J.; Singh, A.; Suntharalingam, P.; Uematsu, M.; Zamora, L. M. (2017). "A reevaluation of the magnitude and impacts of anthropogenic atmospheric nitrogen inputs on the ocean". Global Biogeochemical Cycles. 31 (2): 289. Bibcode:2017GBioC..31..289J. doi:10.1002/2016GB005586. hdl:1874/348077.
- ↑ Bouwman, A. F.; Van Drecht, G.; Knoop, J. M.; Beusen, A. H. W.; Meinardi, C. R. (2005). "Exploring changes in river nitrogen export to the world's oceans". Global Biogeochemical Cycles. 19 (1). Bibcode:2005GBioC..19.1002B. doi:10.1029/2004GB002314.
- ↑ Altieri, Andrew H.; Gedan, Keryn B. (2015). "Climate change and dead zones". Global Change Biology. 21 (4): 1395–1406. Bibcode:2015GCBio..21.1395A. doi:10.1111/gcb.12754. PMID 25385668.
- ↑ 22.0 22.1 Breitburg, Denise; Levin, Lisa A.; Oschlies, Andreas; Grégoire, Marilaure; Chavez, Francisco P.; Conley, Daniel J.; Garçon, Véronique; Gilbert, Denis; Gutiérrez, Dimitri; Isensee, Kirsten; Jacinto, Gil S.; Limburg, Karin E.; Montes, Ivonne; Naqvi, S. W. A.; Pitcher, Grant C.; Rabalais, Nancy N.; Roman, Michael R.; Rose, Kenneth A.; Seibel, Brad A.; Telszewski, Maciej; Yasuhara, Moriaki; Zhang, Jing (2018). "Declining oxygen in the global ocean and coastal waters". Science. 359 (6371): eaam7240. Bibcode:2018Sci...359M7240B. doi:10.1126/science.aam7240. PMID 29301986. S2CID 206657115.
- ↑ 23.0 23.1 Cavicchioli, Ricardo; et al. (2019). "Scientists' warning to humanity: Microorganisms and climate change". Nature Reviews Microbiology. 17 (9): 569–586. doi:10.1038/s41579-019-0222-5. PMC 7136171. PMID 31213707.
- ↑ 24.0 24.1 Hutchins, David A.; Jansson, Janet K.; Remais, Justin V.; Rich, Virginia I.; Singh, Brajesh K.; Trivedi, Pankaj (2019). "Climate change microbiology — problems and perspectives". Nature Reviews Microbiology. 17 (6): 391–396. doi:10.1038/s41579-019-0178-5. PMID 31092905. S2CID 155102440.
- ↑ Stillman, Jonathon H.; Paganini, Adam W. (2015). "Biochemical adaptation to ocean acidification". Journal of Experimental Biology. 218 (12): 1946–1955. doi:10.1242/jeb.115584. PMID 26085671. S2CID 13071345.
- ↑ Bertagnolli, Anthony D.; Stewart, Frank J. (2018). "Microbial niches in marine oxygen minimum zones". Nature Reviews Microbiology. 16 (12): 723–729. doi:10.1038/s41579-018-0087-z. PMID 30250271. S2CID 52811177.
- ↑ Ulloa, O.; Canfield, D. E.; Delong, E. F.; Letelier, R. M.; Stewart, F. J. (2012). "Microbial oceanography of anoxic oxygen minimum zones". Proceedings of the National Academy of Sciences. 109 (40): 15996–16003. Bibcode:2012PNAS..10915996U. doi:10.1073/pnas.1205009109. PMC 3479542. PMID 22967509. S2CID 6630698.
- ↑ 28.0 28.1 Libes, Susan M. (2015). Blue planet: The role of the oceans in nutrient cycling, maintain the atmosphere system, and modulating climate change -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2021-01-20. In: Routledge Handbook of Ocean Resources and Management, Routledge, pages 89–107. .
- ↑ 29.0 29.1 Bush, Martin J. (2020). Climate Change and Renewable Energy. pp. 109–141. doi:10.1007/978-3-030-15424-0_3. ISBN 978-3-030-15423-3. https://books.google.com/books?id=h_60DwAAQBAJ&q=%22Climate+Change+and+Renewable+Energy%22+%22The+Carbon+Cycle%22chapter+%3D+The+Carbon+Cycle&pg=PA109.
- ↑ Rothman, D. H. (2002). "Atmospheric carbon dioxide levels for the last 500 million years". Proceedings of the National Academy of Sciences. 99 (7): 4167–4171. Bibcode:2002PNAS...99.4167R. doi:10.1073/pnas.022055499. PMC 123620. PMID 11904360.
- ↑ Carpinteri, Alberto; Niccolini, Gianni (2019). "Correlation between the Fluctuations in Worldwide Seismicity and Atmospheric Carbon Pollution". Sci. 1: 17. doi:10.3390/sci1010017. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2017-10-16..
- ↑ Rothman, Daniel (January 2015). "Earth's carbon cycle: A mathematical perspective". Bulletin of the American Mathematical Society (in English). 52 (1): 47–64. doi:10.1090/S0273-0979-2014-01471-5. hdl:1721.1/97900. ISSN 0273-0979. Archived from the original on 2021-11-22. Retrieved 2021-09-27.
- ↑ Riebeek, Holli (16 June 2011). "The Carbon Cycle". Earth Observatory. NASA. Archived from the original on 5 March 2016. Retrieved 5 April 2018.
- ↑ McMahon, Sean; Parnell, John (2014). "Weighing the deep continental biosphere". FEMS Microbiology Ecology. 87 (1): 113–120. doi:10.1111/1574-6941.12196. PMID 23991863.
- ↑ Kallmeyer, J.; Pockalny, R.; Adhikari, R. R.; Smith, D. C.; d'Hondt, S. (2012). "Global distribution of microbial abundance and biomass in subseafloor sediment". Proceedings of the National Academy of Sciences. 109 (40): 16213–16216. doi:10.1073/pnas.1203849109. PMC 3479597. PMID 22927371.
- ↑ Schloss, Patrick D.; Girard, Rene A.; Martin, Thomas; Edwards, Joshua; Thrash, J. Cameron (2016). "Status of the Archaeal and Bacterial Census: An Update". mBio. 7 (3). doi:10.1128/mBio.00201-16. PMC 4895100. PMID 27190214.
- ↑ Abreu, Nicole A.; Taga, Michiko E. (2016). "Decoding molecular interactions in microbial communities". FEMS Microbiology Reviews. 40 (5): 648–663. doi:10.1093/femsre/fuw019. PMC 5007284. PMID 27417261.
- ↑ Bosse, Magnus; Heuwieser, Alexander; Heinzel, Andreas; Nancucheo, Ivan; Melo Barbosa Dall'Agnol, Hivana; Lukas, Arno; Tzotzos, George; Mayer, Bernd (2015). "Interaction networks for identifying coupled molecular processes in microbial communities". BioData Mining. 8: 21. doi:10.1186/s13040-015-0054-4. PMC 4502522. PMID 26180552.
- ↑ Braker, Gesche; Dörsch, Peter; Bakken, Lars R. (2012). "Genetic characterization of denitrifier communities with contrasting intrinsic functional traits". FEMS Microbiology Ecology. 79 (2): 542–554. doi:10.1111/j.1574-6941.2011.01237.x. PMID 22092293.
- ↑ 40.0 40.1 Hug, Laura A.; Thomas, Brian C.; Sharon, Itai; Brown, Christopher T.; Sharma, Ritin; Hettich, Robert L.; Wilkins, Michael J.; Williams, Kenneth H.; Singh, Andrea; Banfield, Jillian F. (2016). "Critical biogeochemical functions in the subsurface are associated with bacteria from new phyla and little studied lineages". Environmental Microbiology. 18 (1): 159–173. doi:10.1111/1462-2920.12930. PMID 26033198. Archived from the original on 2021-09-27. Retrieved 2021-09-27.
- ↑ McCarren, J.; Becker, J. W.; Repeta, D. J.; Shi, Y.; Young, C. R.; Malmstrom, R. R.; Chisholm, S. W.; Delong, E. F. (2010). "Microbial community transcriptomes reveal microbes and metabolic pathways associated with dissolved organic matter turnover in the sea". Proceedings of the National Academy of Sciences. 107 (38): 16420–16427. doi:10.1073/pnas.1010732107. PMC 2944720. PMID 20807744.
- ↑ Embree, Mallory; Liu, Joanne K.; Al-Bassam, Mahmoud M.; Zengler, Karsten (2015). "Networks of energetic and metabolic interactions define dynamics in microbial communities". Proceedings of the National Academy of Sciences. 112 (50): 15450–15455. Bibcode:2015PNAS..11215450E. doi:10.1073/pnas.1506034112. PMC 4687543. PMID 26621749.
- ↑ Long, Philip E.; Williams, Kenneth H.; Hubbard, Susan S.; Banfield, Jillian F. (2016). "Microbial Metagenomics Reveals Climate-Relevant Subsurface Biogeochemical Processes". Trends in Microbiology. 24 (8): 600–610. doi:10.1016/j.tim.2016.04.006. PMID 27156744.
- ↑ Eren, A. Murat; Esen, Özcan C.; Quince, Christopher; Vineis, Joseph H.; Morrison, Hilary G.; Sogin, Mitchell L.; Delmont, Tom O. (2015). "Anvi'o: An advanced analysis and visualization platform for 'omics data". PeerJ. 3: e1319. doi:10.7717/peerj.1319. PMC 4614810. PMID 26500826.
- ↑ Alneberg, Johannes; Bjarnason, Brynjar Smári; De Bruijn, Ino; Schirmer, Melanie; Quick, Joshua; Ijaz, Umer Z.; Lahti, Leo; Loman, Nicholas J.; Andersson, Anders F.; Quince, Christopher (2014). "Binning metagenomic contigs by coverage and composition". Nature Methods. 11 (11): 1144–1146. doi:10.1038/nmeth.3103. PMID 25218180. S2CID 24696869.
- ↑ Anantharaman, Karthik; Brown, Christopher T.; Hug, Laura A.; Sharon, Itai; Castelle, Cindy J.; Probst, Alexander J.; Thomas, Brian C.; Singh, Andrea; Wilkins, Michael J.; Karaoz, Ulas; Brodie, Eoin L.; Williams, Kenneth H.; Hubbard, Susan S.; Banfield, Jillian F. (2016). "Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system". Nature Communications. 7: 13219. Bibcode:2016NatCo...713219A. doi:10.1038/ncomms13219. PMC 5079060. PMID 27774985. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License -{zh-cn:互联网档案馆; zh-tw:網際網路檔案館; zh-hk:互聯網檔案館;}-的存檔,存档日期2017-10-16..
- ↑ "Mercury Cycling in the Environment". Wisconsin Water Science Center. United States Geological Survey. 10 January 2013. Archived from the original on 11 April 2021. Retrieved 20 November 2017.
- ↑ Organic contaminants that leave traces : sources, transport and fate. Ifremer. pp. 22–23. ISBN 9782759200139.
- ↑ Galy, Valier; Peucker-Ehrenbrink, Bernhard; Eglinton, Timothy (2015). "Global carbon export from the terrestrial biosphere controlled by erosion". Nature. 521 (7551): 204–207. Bibcode:2015Natur.521..204G. doi:10.1038/nature14400. PMID 25971513. S2CID 205243485.
- ↑ Hedges, J.I; Oades, J.M (1997). "Comparative organic geochemistries of soils and marine sediments". Organic Geochemistry. 27 (7–8): 319–361. doi:10.1016/S0146-6380(97)00056-9.
- ↑ McGuire, 1A. D.; Lukina, N. V. (2007). "Biogeochemical cycles". Northern Eurasia earth science partnership initiative (NEESPI), Science plan overview. Global Planetary Change. 56. pp. 215–234. http://neespi.org/science/NEESPI_SP_chapters/SP_Chapter_3.2.pdf.
- ↑ "Distributed Active Archive Center for Biogeochemical Dynamics". daac.ornl.gov. Oak Ridge National Laboratory. Archived from the original on 11 February 2011. Retrieved 20 November 2017.
Further reading
- Schink, Bernhard; "Microbes: Masters of the Global Element Cycles" pp 33–58. "Metals, Microbes and Minerals: The Biogeochemical Side of Life", pp xiv + 341. Walter de Gruyter, Berlin. DOI 10.1515/9783110589771-002
- Global biogeochemical cycles. London: Academic Press. 1993. ISBN 9780080954707.
- Exley, C (15 September 2003). "A biogeochemical cycle for aluminium?". Journal of Inorganic Biochemistry. 97 (1): 1–7. doi:10.1016/S0162-0134(03)00274-5. PMID 14507454.
- Jacobson, Michael C.; Charlson, Robert J.; Rodhe, Henning; Orians, Gordon H. (2000). Earth system science from biogeochemical cycles to global change (2nd ed.). San Diego, Calif.: Academic Press. ISBN 9780080530642.
- Palmeri, Luca; Barausse, Alberto; Jorgensen, Sven Erik (2013). "12. Biogeochemical cycles". Ecological processes handbook. Boca Raton: Taylor & Francis. ISBN 9781466558489.
- Schink, Bernhard; "Microbes: Masters of the Global Element Cycles" pp 33–58. "Metals, Microbes and Minerals: The Biogeochemical Side of Life", pp xiv + 341. Walter de Gruyter, Berlin. DOI 10.1515/9783110589771-002
= 进一步阅读 = =
- Schink,Bernhard; “ Microbes: Masters of the Global Element cycle”pp 33-58。“金属、微生物和矿物: 生命的生物地球化学方面”,pp xiv + 341。德格鲁伊特,柏林。DOI 10.1515/9783110589771-002
模板:Biogeochemical cycle 模板:Modelling ecosystems
Category:Geochemistry Category:Biogeography
类别: 地球化学类别: 生物地理学
This page was moved from wikipedia:en:Biogeochemical cycle. Its edit history can be viewed at 生物地球化学循环/edithistory
- 含有受损文件链接的页面
- 有参考文献错误的页面
- Webarchive模板wayback链接
- CS1 errors: missing periodical
- Pages containing cite templates with deprecated parameters
- CS1 English-language sources (en)
- Articles with short description
- Articles with hatnote templates targeting a nonexistent page
- AC with 0 elements
- Pages with red-linked authority control categories
- Biogeochemical cycle
- Geochemistry
- Biogeography
- 待整理页面