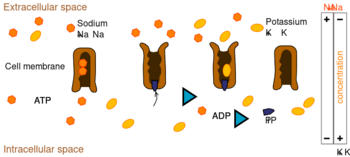“膜电位”的版本间的差异
小 |
小 |
||
| 第17行: | 第17行: | ||
图注:''Key'': {{fontcolor|blue|Blue}} 五边形——钠离子;{{fontcolor|purple|Purple}} 正方形——钾离子;{{fontcolor|yellow|gray|Yellow}} 圆形——氯离子;{{fontcolor|darkorange|Orange}} 矩形——膜不通透的阴离子(它们来源广泛,包括蛋白质)。带箭头的大 {{fontcolor|purple|purple}} 的结构表示跨膜钾离子通道和钾离子的净流动方向。|链接=Special:FilePath/Basis_of_Membrane_Potential2.png]] | 图注:''Key'': {{fontcolor|blue|Blue}} 五边形——钠离子;{{fontcolor|purple|Purple}} 正方形——钾离子;{{fontcolor|yellow|gray|Yellow}} 圆形——氯离子;{{fontcolor|darkorange|Orange}} 矩形——膜不通透的阴离子(它们来源广泛,包括蛋白质)。带箭头的大 {{fontcolor|purple|purple}} 的结构表示跨膜钾离子通道和钾离子的净流动方向。|链接=Special:FilePath/Basis_of_Membrane_Potential2.png]] | ||
| − | + | 膜电位(也叫跨膜电位或膜电压)是生物细胞内部和外部的电位差。也就是说,只要没有获得动能或产生辐射,电荷从细胞内环境移动到细胞外环境与从外部移动内部所需的能量是不同的。电荷的浓度梯度直接决定这种能量要求。相对细胞外部,典型的膜电位值,通常以毫伏(表示为 mV)为单位,处于 -80 mV 到 -40 mV 的范围。 | |
| − | + | 所有的动物细胞都被内嵌蛋白质的脂质双分子层组成的膜所包围。这种膜既对电荷绝缘,又阻挡了离子的扩散运动。被称为离子转运蛋白(ion transporter)或离子泵(ion pump)的跨膜蛋白,主动地对离子跨膜转运从而建立跨膜浓度梯度,而离子通道允许离子沿着浓度梯度跨膜移动。离子泵和离子通道在电学上相当于一组插入膜中的电池和电阻,因此在膜的两侧产生电压。 | |
| − | + | 几乎所有的细胞质膜都存在一个跨膜电位,内部通常相对于外部是负电位 <ref name=":0">{{Cite book|title=Molecular biology of the cell|last=Bruce|first=Alberts|isbn=9780815344322|edition=Sixth|location=New York, NY|oclc=887605755|date = 2014-11-18}}</ref>。膜电位有两个基本功能。首先,它允许细胞像电池一样工作,提供动力来操作嵌在膜中的各种“分子设备”<ref name=":1">{{Cite journal|last1=Abdul Kadir|first1=Lina|last2=Stacey|first2=Michael|last3=Barrett-Jolley|first3=Richard|date=2018|title=Emerging Roles of the Membrane Potential: Action Beyond the Action Potential|journal=Frontiers in Physiology|language=English|volume=9|doi=10.3389/fphys.2018.01661|pmid=30519193|issn=1664-042X|doi-access=free}}</ref> 。其次,在神经元和肌肉细胞等电兴奋性细胞中,它可用来在细胞的不同部位之间传递信号。信号是通过在膜的某一点打开或关闭离子通道而产生的,从而引起膜电位的局部变化。这种电场的变化可以被膜上相邻或更远的离子通道迅速检测到。这些离子通道可以随着电位的变化而打开或关闭,重新产生信号。 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
In non-excitable cells, and in excitable cells in their baseline states, the membrane potential is held at a relatively stable value, called the [[resting potential]]. For neurons, resting potential is defined as ranging from –80 to –70 millivolts; that is, the interior of a cell has a negative baseline voltage of a bit less than one-tenth of a volt. The opening and closing of ion channels can induce a departure from the resting potential. This is called a [[depolarization]] if the interior voltage becomes less negative (say from –70 mV to –60 mV), or a [[hyperpolarization (biology)|hyperpolarization]] if the interior voltage becomes more negative (say from –70 mV to –80 mV). In excitable cells, a sufficiently large depolarization can evoke an [[action potential]], in which the membrane potential changes rapidly and significantly for a short time (on the order of 1 to 100 milliseconds), often reversing its polarity. Action potentials are generated by the activation of certain [[voltage-gated ion channel]]s. | In non-excitable cells, and in excitable cells in their baseline states, the membrane potential is held at a relatively stable value, called the [[resting potential]]. For neurons, resting potential is defined as ranging from –80 to –70 millivolts; that is, the interior of a cell has a negative baseline voltage of a bit less than one-tenth of a volt. The opening and closing of ion channels can induce a departure from the resting potential. This is called a [[depolarization]] if the interior voltage becomes less negative (say from –70 mV to –60 mV), or a [[hyperpolarization (biology)|hyperpolarization]] if the interior voltage becomes more negative (say from –70 mV to –80 mV). In excitable cells, a sufficiently large depolarization can evoke an [[action potential]], in which the membrane potential changes rapidly and significantly for a short time (on the order of 1 to 100 milliseconds), often reversing its polarity. Action potentials are generated by the activation of certain [[voltage-gated ion channel]]s. | ||
| − | + | 在非兴奋性细胞以及处于基线状态的兴奋性细胞,膜电位保持在一个相对稳定的值,称为静息电位。对于神经元,静息电位的定义为 -80 到 -70 mV;也就是说,细胞内部的负基线电压略低于十分之一伏特。离子通道的开启和关闭可以导致静息电位的偏离。如果内部电压变得不那么负(比如从-70 mV 到-60 mV) ,这就叫去极化; 如果内部电压变得更负(比如从-70 mV 到-80 mV) ,这就叫去极化超极化。在可兴奋的细胞中,足够大去极化可以激发动作电位,在这个动作电位中,膜电位在短时间内迅速而显著地改变(大约1到100毫秒) ,经常反转其极性。动作电位是通过激活某些电压门控离子通道而产生的。 | |
In neurons, the factors that influence the membrane potential are diverse. They include numerous types of ion channels, some of which are chemically gated and some of which are voltage-gated. Because voltage-gated ion channels are controlled by the membrane potential, while the membrane potential itself is influenced by these same ion channels, feedback loops that allow for complex temporal dynamics arise, including oscillations and regenerative events such as action potentials. | In neurons, the factors that influence the membrane potential are diverse. They include numerous types of ion channels, some of which are chemically gated and some of which are voltage-gated. Because voltage-gated ion channels are controlled by the membrane potential, while the membrane potential itself is influenced by these same ion channels, feedback loops that allow for complex temporal dynamics arise, including oscillations and regenerative events such as action potentials. | ||
| 第200行: | 第194行: | ||
其中 | 其中 | ||
| − | * | + | *''E''<sub>eq,K<sup>+</sup></sub> 是钾的平衡电位,用伏特为单位 |
| − | * | + | *''R'' 为通用气体常数,等于 8.314 [[joule]]s·K<sup>−1</sup>·mol<sup>−1</sup> |
| − | * | + | *''T'' 为绝对温度,以 K 为单位 in [[kelvin]]s (= K = degrees Celsius + 273.15) |
| − | * z | + | *''z'' 反应中所涉及的离子的基本电荷数 |
| − | * | + | *''F'' 是法拉第常数,等于 96,485 [[coulomb]]s·mol<sup>−1</sup> is the [[Faraday constant]], equal to 96,485 [[coulomb]]s·mol<sup>−1</sup> or J·V<sup>−1</sup>·mol<sup>−1</sup> |
| + | *[K<sup>+</sup>]<sub>o</sub> 为钾离子的胞外浓度 measured in [[Mole (unit)|mol]]·m<sup>−3</sup> or mmol·l<sup>−1</sup> | ||
| + | *[K<sup>+</sup>]<sub>i</sub> is 为钾离子的胞内浓度, | ||
| − | + | 即使两种离子具有相同的电荷(即 K<sup>+</sup> and Na<sup>+</sup> ),只要胞内外浓度不同,它们仍然具有非常不同的平衡电位。以神经元中钾和钠的平衡电位为例,钾离子在胞内为 140 mM,胞外 5 mM,平衡电位为 -84 mV;而钠离子胞内约 12 mM,胞外 140 mM,平衡电位 ''E''<sub>Na</sub> 约为 +66 mV <ref name=":0" group="note">Note that the signs of ''E''<sub>Na</sub> and ''E''<sub>K</sub> are opposite. This is because the concentration gradient for potassium is directed out of the cell, while the concentration gradient for sodium is directed into the cell. Membrane potentials are defined relative to the exterior of the cell; thus, a potential of −70 mV implies that the interior of the cell is negative relative to the exterior.</ref>。 | |
| − | + | ===发育中膜电位的变化=== | |
| − | |||
| − | = | ||
A [[neuron]]'s resting membrane potential actually changes during the [[Neural development|development]] of an organism. In order for a neuron to eventually adopt its full adult function, its potential must be tightly regulated during development. As an organism progresses through development the resting membrane potential becomes more negative.<ref name=":7">{{Cite journal|last1=Sanes|first1=Dan H.|last2=Takács|first2=Catherine|date=1993-06-01|title=Activity-dependent Refinement of Inhibitory Connections|journal=European Journal of Neuroscience|language=en|volume=5|issue=6|pages=570–574|doi=10.1111/j.1460-9568.1993.tb00522.x|pmid=8261131|s2cid=30714579|issn=1460-9568}}</ref> [[Neuroglia|Glial cells]] are also differentiating and proliferating as development progresses in the [[brain]].<ref name=":8">{{Cite journal|last1=KOFUJI|first1=P.|last2=NEWMAN|first2=E. A.|date=2004-01-01|journal=Neuroscience|volume=129|issue=4|pages=1045–1056|doi=10.1016/j.neuroscience.2004.06.008|issn=0306-4522| pmc=2322935 |pmid=15561419|title=Potassium buffering in the central nervous system}}</ref> The addition of these glial cells increases the organism's ability to regulate extracellular [[potassium]]. The drop in extracellular potassium can lead to a decrease in membrane potential of 35 mV.<ref name=":9">{{Cite book|title=Development of the nervous system|last1=Sanes|first1=Dan H.|last2=Reh|first2=Thomas A|date=2012-01-01|publisher=Elsevier Academic Press|isbn=9780080923208|pages=211–214|oclc=762720374|edition=Third}}</ref> | A [[neuron]]'s resting membrane potential actually changes during the [[Neural development|development]] of an organism. In order for a neuron to eventually adopt its full adult function, its potential must be tightly regulated during development. As an organism progresses through development the resting membrane potential becomes more negative.<ref name=":7">{{Cite journal|last1=Sanes|first1=Dan H.|last2=Takács|first2=Catherine|date=1993-06-01|title=Activity-dependent Refinement of Inhibitory Connections|journal=European Journal of Neuroscience|language=en|volume=5|issue=6|pages=570–574|doi=10.1111/j.1460-9568.1993.tb00522.x|pmid=8261131|s2cid=30714579|issn=1460-9568}}</ref> [[Neuroglia|Glial cells]] are also differentiating and proliferating as development progresses in the [[brain]].<ref name=":8">{{Cite journal|last1=KOFUJI|first1=P.|last2=NEWMAN|first2=E. A.|date=2004-01-01|journal=Neuroscience|volume=129|issue=4|pages=1045–1056|doi=10.1016/j.neuroscience.2004.06.008|issn=0306-4522| pmc=2322935 |pmid=15561419|title=Potassium buffering in the central nervous system}}</ref> The addition of these glial cells increases the organism's ability to regulate extracellular [[potassium]]. The drop in extracellular potassium can lead to a decrease in membrane potential of 35 mV.<ref name=":9">{{Cite book|title=Development of the nervous system|last1=Sanes|first1=Dan H.|last2=Reh|first2=Thomas A|date=2012-01-01|publisher=Elsevier Academic Press|isbn=9780080923208|pages=211–214|oclc=762720374|edition=Third}}</ref> | ||
| − | + | 神经元的静息膜电位在生物体发育过程中会发生变化。为了让神经元最终发挥其完整的成年期功能,它的潜能必须在发育过程中受到严格的调控。随着生物体的发育,静息膜电位变得更负 <ref name=":7" /> 。随着脑的发育,神经胶质细胞也在分化和增殖 <ref name=":8" />。增加的神经胶质细胞增加了机体调节细胞外钾的能力。细胞外液中的钾的下降可导致膜电位下降 35 mV <ref name=":9" />。 | |
| − | === | + | === 细胞兴奋性=== |
| − | {{ | + | 细胞兴奋性(Cell excitability)是各种组织中的细胞反应所必需的细胞膜电位变化。细胞兴奋性是早期胚胎发生过程中诱导的一种特性 <ref name=":10">{{Cite journal|last=Tosti|first=Elisabetta|date=2010-06-28|title=Dynamic roles of ion currents in early development|journal=Molecular Reproduction and Development|volume=77|issue=10|pages=856–867|doi=10.1002/mrd.21215|pmid=20586098|s2cid=38314187|issn=1040-452X|doi-access=free}}</ref>。细胞兴奋性也被定义为引起反应的容易程度 <ref name=":11">{{Cite journal|last1=Boyet|first1=M.R.|last2=Jewell|first2=B.R.|date=1981|title=Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart|journal=Progress in Biophysics and Molecular Biology|volume=36|issue=1|pages=1–52|doi=10.1016/0079-6107(81)90003-1|pmid=7001542|issn=0079-6107|doi-access=free}}</ref> 。静息电位和阈电位是细胞兴奋性的基础,这些过程是细胞剂量电位和动作电位产生的基础。 |
| − | + | 细胞兴奋性最重要的调节因子是细胞外电解质浓度(即 Na<sup>+</sup>, K<sup>+</sup>, [[Calcium metabolism|Ca<sup>2+</sup>]], Cl<sup>−</sup>, [[Magnesium in biology|Mg<sup>2+</sup>]])及其相关蛋白。调节细胞兴奋性的重要蛋白质是电压门控离子通道、离子转运蛋白(如钠钾 ATP 酶、镁转运蛋白、酸碱转运蛋白)、膜受体和超极化激活的环核苷酸门控通道 <ref name=":12">{{Cite journal|last1=Spinelli|first1=Valentina|last2=Sartiani|first2=Laura|last3=Mugelli|first3=Alessandro|last4=Romanelli|first4=Maria Novella|last5=Cerbai|first5=Elisabetta|date=2018|title=Hyperpolarization-activated cyclic-nucleotide-gated channels: pathophysiological, developmental, and pharmacological insights into their function in cellular excitability|journal=Canadian Journal of Physiology and Pharmacology|volume=96|issue=10|pages=977–984|doi=10.1139/cjpp-2018-0115|pmid=29969572|issn=0008-4212|hdl=1807/90084|hdl-access=free}}</ref>。例如,钾离子通道和钙敏感受体是神经元、心肌细胞和星形胶质细胞等其他兴奋性细胞的兴奋性的重要调节因子<ref name=":13">{{Cite journal|last1=Jones|first1=Brian L.|last2=Smith|first2=Stephen M.|date=2016-03-30|title=Calcium-Sensing Receptor: A Key Target for Extracellular Calcium Signaling in Neurons|journal=Frontiers in Physiology|volume=7|page=116|doi=10.3389/fphys.2016.00116|pmid=27065884|pmc=4811949|issn=1664-042X|doi-access=free}}</ref> 。钙离子也是可兴奋细胞信号转导中最重要的第二信使。突触受体的激活产生神经元兴奋性的长期改变 <ref name=":14">{{Cite journal|last1=Debanne|first1=Dominique|last2=Inglebert|first2=Yanis|last3=Russier|first3=Michaël|date=2019|title=Plasticity of intrinsic neuronal excitability|journal=Current Opinion in Neurobiology|language=en|volume=54|pages=73–82|doi=10.1016/j.conb.2018.09.001|pmid=30243042|s2cid=52812190|url=https://hal-amu.archives-ouvertes.fr/hal-01963474/file/Debannne-Russier-2019.pdf}}</ref>。甲状腺激素、肾上腺激素和其他激素也调节细胞的兴奋性,例如,孕酮和雌激素调节子宫平滑肌细胞的兴奋性。 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Many cell types are considered to have an excitable membrane. Excitable cells are neurons, myocytes (cardiac, skeletal, [[Smooth muscle|smooth]]), vascular [[Endothelium|endothelial cells]], [[pericyte]]s, [[juxtaglomerular cell]]s, [[Interstitial cell of Cajal|interstitial cells of Cajal]], many types of [[Epithelium|epithelial cells]] (e.g. [[beta cell]]s, [[alpha cell]]s, [[delta cell]]s, [[enteroendocrine cell]]s, [[Neuroendocrine_cell#Pulmonary_neuroendocrine_cells|pulmonary neuroendocrine cells]], [[pinealocyte]]s), [[glia]]l cells (e.g. astrocytes), [[mechanoreceptor]] cells (e.g. [[hair cell]]s and [[Merkel cell]]s), [[chemoreceptor]] cells (e.g. [[glomus cell]]s, [[taste receptor]]s), some [[plant cells]] and possibly [[White blood cell|immune cells]].<ref name=":15">{{Cite journal|last1=Davenport|first1=Bennett|last2=Li|first2=Yuan|last3=Heizer|first3=Justin W.|last4=Schmitz|first4=Carsten|last5=Perraud|first5=Anne-Laure|date=2015-07-23|title=Signature Channels of Excitability no More: L-Type Channels in Immune Cells|journal=Frontiers in Immunology|volume=6|page=375|doi=10.3389/fimmu.2015.00375|pmid=26257741|pmc=4512153|issn=1664-3224|doi-access=free}}</ref> Astrocytes display a form of non-electrical excitability based on intracellular calcium variations related to the expression of several receptors through which they can detect the synaptic signal. In neurons, there are different membrane properties in some portions of the cell, for example, dendritic excitability endows neurons with the capacity for coincidence detection of spatially separated inputs.<ref name=":16">{{Cite journal|last=Sakmann|first=Bert|date=2017-04-21|title=From single cells and single columns to cortical networks: dendritic excitability, coincidence detection and synaptic transmission in brain slices and brains|journal=Experimental Physiology|volume=102|issue=5|pages=489–521|doi=10.1113/ep085776|pmid=28139019|pmc=5435930|issn=0958-0670|doi-access=free}}</ref> | Many cell types are considered to have an excitable membrane. Excitable cells are neurons, myocytes (cardiac, skeletal, [[Smooth muscle|smooth]]), vascular [[Endothelium|endothelial cells]], [[pericyte]]s, [[juxtaglomerular cell]]s, [[Interstitial cell of Cajal|interstitial cells of Cajal]], many types of [[Epithelium|epithelial cells]] (e.g. [[beta cell]]s, [[alpha cell]]s, [[delta cell]]s, [[enteroendocrine cell]]s, [[Neuroendocrine_cell#Pulmonary_neuroendocrine_cells|pulmonary neuroendocrine cells]], [[pinealocyte]]s), [[glia]]l cells (e.g. astrocytes), [[mechanoreceptor]] cells (e.g. [[hair cell]]s and [[Merkel cell]]s), [[chemoreceptor]] cells (e.g. [[glomus cell]]s, [[taste receptor]]s), some [[plant cells]] and possibly [[White blood cell|immune cells]].<ref name=":15">{{Cite journal|last1=Davenport|first1=Bennett|last2=Li|first2=Yuan|last3=Heizer|first3=Justin W.|last4=Schmitz|first4=Carsten|last5=Perraud|first5=Anne-Laure|date=2015-07-23|title=Signature Channels of Excitability no More: L-Type Channels in Immune Cells|journal=Frontiers in Immunology|volume=6|page=375|doi=10.3389/fimmu.2015.00375|pmid=26257741|pmc=4512153|issn=1664-3224|doi-access=free}}</ref> Astrocytes display a form of non-electrical excitability based on intracellular calcium variations related to the expression of several receptors through which they can detect the synaptic signal. In neurons, there are different membrane properties in some portions of the cell, for example, dendritic excitability endows neurons with the capacity for coincidence detection of spatially separated inputs.<ref name=":16">{{Cite journal|last=Sakmann|first=Bert|date=2017-04-21|title=From single cells and single columns to cortical networks: dendritic excitability, coincidence detection and synaptic transmission in brain slices and brains|journal=Experimental Physiology|volume=102|issue=5|pages=489–521|doi=10.1113/ep085776|pmid=28139019|pmc=5435930|issn=0958-0670|doi-access=free}}</ref> | ||
| − | + | 许多细胞类型被认为具有兴奋性膜。兴奋性细胞包括神经元、肌细胞(心肌细胞、骨骼肌细胞、平滑肌细胞)、血管内皮细胞、周细胞、肾小球旁细胞、Cajal 间质细胞、多种类型的上皮细胞(如 β 细胞、α 细胞、δ 细胞、肠内分泌细胞、肺神经内分泌细胞、松果体细胞)、胶质细胞(例如星形胶质细胞)、机械感觉受体细胞(例如毛细胞和 Merkel 细胞)、化学感觉受体细胞(例如血管球细胞、味觉受体细胞)、一些植物细胞,可能还有免疫细胞 <ref name=":15" />。星形胶质细胞表现出一种非电兴奋性,这种兴奋性是基于细胞内钙离子的变化,这种变化与几个受体的表达有关,通过这些受体它们可以检测到突触信号。在神经元中,细胞的某些部分具有不同的膜特性,例如,树突的兴奋性赋予神经元对空间上分离的输入信号进行重合检测的能力 <ref name=":16" />。 | |
| − | ===Equivalent | + | ===Equivalent circuit等效电路=== |
[[File:Cell membrane equivalent circuit.svg|thumb|right|350px|Equivalent circuit for a patch of membrane, consisting of a fixed capacitance in parallel with four pathways each containing a battery in series with a variable conductance膜片的等效电路,由固定电容组成,并联四条通路,每条通路串联一个电池,电导率可变。|链接=Special:FilePath/Cell_membrane_equivalent_circuit.svg]] | [[File:Cell membrane equivalent circuit.svg|thumb|right|350px|Equivalent circuit for a patch of membrane, consisting of a fixed capacitance in parallel with four pathways each containing a battery in series with a variable conductance膜片的等效电路,由固定电容组成,并联四条通路,每条通路串联一个电池,电导率可变。|链接=Special:FilePath/Cell_membrane_equivalent_circuit.svg]] | ||
Electrophysiologists model the effects of ionic concentration differences, ion channels, and membrane capacitance in terms of an [[equivalent circuit]], which is intended to represent the electrical properties of a small patch of membrane. The equivalent circuit consists of a capacitor in parallel with four pathways each consisting of a battery in series with a variable conductance. The capacitance is determined by the properties of the lipid bilayer, and is taken to be fixed. Each of the four parallel pathways comes from one of the principal ions, sodium, potassium, chloride, and calcium. The voltage of each ionic pathway is determined by the concentrations of the ion on each side of the membrane; see the [[Membrane potential#Reversal potential|Reversal potential]] section above. The conductance of each ionic pathway at any point in time is determined by the states of all the ion channels that are potentially permeable to that ion, including leakage channels, ligand-gated channels, and voltage-gated ion channels. | Electrophysiologists model the effects of ionic concentration differences, ion channels, and membrane capacitance in terms of an [[equivalent circuit]], which is intended to represent the electrical properties of a small patch of membrane. The equivalent circuit consists of a capacitor in parallel with four pathways each consisting of a battery in series with a variable conductance. The capacitance is determined by the properties of the lipid bilayer, and is taken to be fixed. Each of the four parallel pathways comes from one of the principal ions, sodium, potassium, chloride, and calcium. The voltage of each ionic pathway is determined by the concentrations of the ion on each side of the membrane; see the [[Membrane potential#Reversal potential|Reversal potential]] section above. The conductance of each ionic pathway at any point in time is determined by the states of all the ion channels that are potentially permeable to that ion, including leakage channels, ligand-gated channels, and voltage-gated ion channels. | ||
| 第301行: | 第288行: | ||
突触后电位是兴奋性电位还是抑制性电位取决于该电流离子的翻转电位,以及细胞激发动作电位的阈值(大约-50mV)。如果突触后电流超过阈值,如典型的钠离子电流,则被认为是兴奋性的。翻转电位。低于阈值的翻转电位,如典型的 k + 电流,被认为是抑制电流。一个翻转电位高于静息电位阈值但低于阈值的电流本身不会引起动作电位,但是会产生阈值以下的膜电位振荡。因此,作用于开放 Na + 通道的神经递质产生兴奋性突触后电位(epsp) ,而作用于开放 k + 或 Cl-通道的神经递质通常产生抑制性突触后电位(ipsp)。当多种类型的通道在同一时间段内开放时,它们的突触后电位相加。 | 突触后电位是兴奋性电位还是抑制性电位取决于该电流离子的翻转电位,以及细胞激发动作电位的阈值(大约-50mV)。如果突触后电流超过阈值,如典型的钠离子电流,则被认为是兴奋性的。翻转电位。低于阈值的翻转电位,如典型的 k + 电流,被认为是抑制电流。一个翻转电位高于静息电位阈值但低于阈值的电流本身不会引起动作电位,但是会产生阈值以下的膜电位振荡。因此,作用于开放 Na + 通道的神经递质产生兴奋性突触后电位(epsp) ,而作用于开放 k + 或 Cl-通道的神经递质通常产生抑制性突触后电位(ipsp)。当多种类型的通道在同一时间段内开放时,它们的突触后电位相加。 | ||
| − | ==Other values== | + | ==Other values 其他值== |
| − | + | 从生物物理学的观点来看,静息膜电位不过是细胞未受刺激时主导的细胞膜通透性所产生的膜电位。上面的加权平均方程总是适用的,但是下面的方法可能更容易可视化。在任何给定的时刻,离子有两个因素决定其对细胞膜电位的影响: | |
| − | + | #离子的驱动力 | |
| − | + | #离子的渗透性 | |
| − | # | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | 如果驱动力很大,那么离子就会被“推”过膜。如果渗透性很高,离子就更容易跨膜扩散。 | |
*'''Driving force''' is the net electrical force available to move that ion across the membrane. It is calculated as the difference between the voltage that the ion "wants" to be at (its equilibrium potential) and the actual membrane potential (''E''<sub>m</sub>). So, in formal terms, the driving force for an ion = ''E''<sub>m</sub> - ''E''<sub>ion</sub> | *'''Driving force''' is the net electrical force available to move that ion across the membrane. It is calculated as the difference between the voltage that the ion "wants" to be at (its equilibrium potential) and the actual membrane potential (''E''<sub>m</sub>). So, in formal terms, the driving force for an ion = ''E''<sub>m</sub> - ''E''<sub>ion</sub> | ||
| 第334行: | 第314行: | ||
离子越多,预测膜电位就越复杂。然而,这可以用 Goldman-Hodgkin-Katz 方程或加权平均数方程来实现。通过在任意时刻输入离子的浓度梯度和渗透率,就可以在那个时刻测定膜电位。方程的意思是,在任何时候,膜电位的值都是所有离子平衡势的加权平均数。“权重”是离子在膜上的相对渗透性。 | 离子越多,预测膜电位就越复杂。然而,这可以用 Goldman-Hodgkin-Katz 方程或加权平均数方程来实现。通过在任意时刻输入离子的浓度梯度和渗透率,就可以在那个时刻测定膜电位。方程的意思是,在任何时候,膜电位的值都是所有离子平衡势的加权平均数。“权重”是离子在膜上的相对渗透性。 | ||
| − | ==Effects and implications== | + | ==Effects and implications 效应与意义== |
While cells expend energy to transport ions and establish a transmembrane potential, they use this potential in turn to transport other ions and metabolites such as sugar. The transmembrane potential of the [[mitochondrial membrane|mitochondria]] drives the production of [[Adenosine triphosphate|ATP]], which is the common currency of biological energy. | While cells expend energy to transport ions and establish a transmembrane potential, they use this potential in turn to transport other ions and metabolites such as sugar. The transmembrane potential of the [[mitochondrial membrane|mitochondria]] drives the production of [[Adenosine triphosphate|ATP]], which is the common currency of biological energy. | ||
| − | + | 当细胞消耗能量来转运离子并建立膜电位时,它们反过来利用这种势能来转运其他离子和代谢物,如糖。线粒体的膜电位驱动 ATP 的产生,ATP 是生物能量的通货。 | |
Cells may draw on the energy they store in the resting potential to drive action potentials or other forms of excitation. These changes in the membrane potential enable communication with other cells (as with action potentials) or initiate changes inside the cell, which happens in an [[Ovum|egg]] when it is [[fertilization|fertilized]] by a [[sperm]]. | Cells may draw on the energy they store in the resting potential to drive action potentials or other forms of excitation. These changes in the membrane potential enable communication with other cells (as with action potentials) or initiate changes inside the cell, which happens in an [[Ovum|egg]] when it is [[fertilization|fertilized]] by a [[sperm]]. | ||
| − | + | 细胞可以利用它们在静息电位中储存的能量来驱动动作电位或其他形式的兴奋。膜电位的这些变化可以与其他细胞交流(如动作电位),或产生在细胞内反应,比如卵细胞和精子受精时发生的。 | |
In neuronal cells, an action potential begins with a rush of sodium ions into the cell through sodium channels, resulting in depolarization, while recovery involves an outward rush of potassium through potassium channels. Both of these fluxes occur by [[passive transport|passive diffusion]]. | In neuronal cells, an action potential begins with a rush of sodium ions into the cell through sodium channels, resulting in depolarization, while recovery involves an outward rush of potassium through potassium channels. Both of these fluxes occur by [[passive transport|passive diffusion]]. | ||
| − | + | 在神经细胞,一个动作电位开始于钠离子通过钠离子通道涌入细胞,导致去极化,而恢复过程中钾离子通过钾离子通道向外涌入。这两种流向都是通过被动扩散产生的。 | |
==See also== | ==See also== | ||
2022年5月22日 (日) 22:26的版本
此词条由神经系统中的动力学模型读书会词条梳理志愿者 okxy 翻译审校,未经专家审核,带来阅读不便,请见谅。
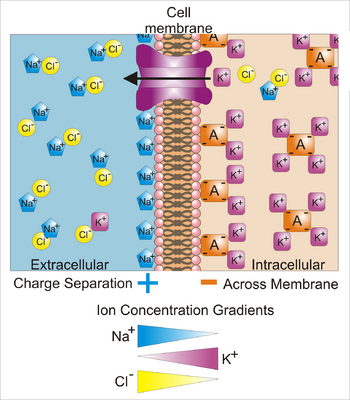
膜电位(也叫跨膜电位或膜电压)是生物细胞内部和外部的电位差。也就是说,只要没有获得动能或产生辐射,电荷从细胞内环境移动到细胞外环境与从外部移动内部所需的能量是不同的。电荷的浓度梯度直接决定这种能量要求。相对细胞外部,典型的膜电位值,通常以毫伏(表示为 mV)为单位,处于 -80 mV 到 -40 mV 的范围。
所有的动物细胞都被内嵌蛋白质的脂质双分子层组成的膜所包围。这种膜既对电荷绝缘,又阻挡了离子的扩散运动。被称为离子转运蛋白(ion transporter)或离子泵(ion pump)的跨膜蛋白,主动地对离子跨膜转运从而建立跨膜浓度梯度,而离子通道允许离子沿着浓度梯度跨膜移动。离子泵和离子通道在电学上相当于一组插入膜中的电池和电阻,因此在膜的两侧产生电压。
几乎所有的细胞质膜都存在一个跨膜电位,内部通常相对于外部是负电位 [1]。膜电位有两个基本功能。首先,它允许细胞像电池一样工作,提供动力来操作嵌在膜中的各种“分子设备”[2] 。其次,在神经元和肌肉细胞等电兴奋性细胞中,它可用来在细胞的不同部位之间传递信号。信号是通过在膜的某一点打开或关闭离子通道而产生的,从而引起膜电位的局部变化。这种电场的变化可以被膜上相邻或更远的离子通道迅速检测到。这些离子通道可以随着电位的变化而打开或关闭,重新产生信号。
In non-excitable cells, and in excitable cells in their baseline states, the membrane potential is held at a relatively stable value, called the resting potential. For neurons, resting potential is defined as ranging from –80 to –70 millivolts; that is, the interior of a cell has a negative baseline voltage of a bit less than one-tenth of a volt. The opening and closing of ion channels can induce a departure from the resting potential. This is called a depolarization if the interior voltage becomes less negative (say from –70 mV to –60 mV), or a hyperpolarization if the interior voltage becomes more negative (say from –70 mV to –80 mV). In excitable cells, a sufficiently large depolarization can evoke an action potential, in which the membrane potential changes rapidly and significantly for a short time (on the order of 1 to 100 milliseconds), often reversing its polarity. Action potentials are generated by the activation of certain voltage-gated ion channels.
在非兴奋性细胞以及处于基线状态的兴奋性细胞,膜电位保持在一个相对稳定的值,称为静息电位。对于神经元,静息电位的定义为 -80 到 -70 mV;也就是说,细胞内部的负基线电压略低于十分之一伏特。离子通道的开启和关闭可以导致静息电位的偏离。如果内部电压变得不那么负(比如从-70 mV 到-60 mV) ,这就叫去极化; 如果内部电压变得更负(比如从-70 mV 到-80 mV) ,这就叫去极化超极化。在可兴奋的细胞中,足够大去极化可以激发动作电位,在这个动作电位中,膜电位在短时间内迅速而显著地改变(大约1到100毫秒) ,经常反转其极性。动作电位是通过激活某些电压门控离子通道而产生的。
In neurons, the factors that influence the membrane potential are diverse. They include numerous types of ion channels, some of which are chemically gated and some of which are voltage-gated. Because voltage-gated ion channels are controlled by the membrane potential, while the membrane potential itself is influenced by these same ion channels, feedback loops that allow for complex temporal dynamics arise, including oscillations and regenerative events such as action potentials.
在神经元中,影响膜电位的因素是多种多样的。它们包括许多类型的离子通道,其中一些是化学门控和一些是电压门控。由于电压门控离子通道是由膜电位控制的,而膜电位本身也受到这些相同离子通道的影响,因此产生了允许复杂时间动力学的反馈回路,包括振荡和再生事件,如动作电位。
Physical basis物理基础
The membrane potential in a cell derives ultimately from two factors: electrical force and diffusion. Electrical force arises from the mutual attraction between particles with opposite electrical charges (positive and negative) and the mutual repulsion between particles with the same type of charge (both positive or both negative). Diffusion arises from the statistical tendency of particles to redistribute from regions where they are highly concentrated to regions where the concentration is low.
细胞中的膜电位最终来自于两个因素: 电力和扩散。电力产生于带有相反电荷的粒子之间的相互吸引(正电荷和负电荷)和带有相同类型电荷的粒子之间的相互斥力(正电荷和负电荷都有)。扩散源于粒子从高度集中的区域向低浓度区域重新分布的统计趋势。
Voltage

Voltage, which is synonymous with difference in electrical potential, is the ability to drive an electric current across a resistance. Indeed, the simplest definition of a voltage is given by Ohm's law: V=IR, where V is voltage, I is current and R is resistance. If a voltage source such as a battery is placed in an electrical circuit, the higher the voltage of the source the greater the amount of current that it will drive across the available resistance. The functional significance of voltage lies only in potential differences between two points in a circuit. The idea of a voltage at a single point is meaningless. It is conventional in electronics to assign a voltage of zero to some arbitrarily chosen element of the circuit, and then assign voltages for other elements measured relative to that zero point. There is no significance in which element is chosen as the zero point—the function of a circuit depends only on the differences not on voltages per se. However, in most cases and by convention, the zero level is most often assigned to the portion of a circuit that is in contact with ground.
电压是电势差的同义词,是驱动电流通过电阻的能力。事实上,电压最简单的定义是由欧姆定律给出的: v = IR,其中 v 是电压,i 是电流,r 是电阻。如果在电路中放置电压源(如电池) ,则电压源的电压越高,通过可用电阻的电流就越大。电压的功能意义仅在于电路中两点之间的电位差。单点电压的概念是没有意义的。在电子学中,通常的做法是给电路中任意选择的元件赋予零电压,然后给相对于该零点测量的其他元件赋予电压。选择哪个元件作为零点没有意义ーー电路的功能只取决于差值,而不取决于电压本身。然而,在大多数情况下,按照惯例,零电平最常被分配到与地接触的电路部分。
The same principle applies to voltage in cell biology. In electrically active tissue, the potential difference between any two points can be measured by inserting an electrode at each point, for example one inside and one outside the cell, and connecting both electrodes to the leads of what is in essence a specialized voltmeter. By convention, the zero potential value is assigned to the outside of the cell and the sign of the potential difference between the outside and the inside is determined by the potential of the inside relative to the outside zero.
同样的原理也适用于细胞生物学中的电压。在电活性组织中,任何两个点之间的电位差都可以通过在每个点插入一个电极来测量,例如在细胞内部和细胞外部插入一个电极,然后将两个电极连接到本质上是一个专门的电压表的导线上。按照惯例,电位零值被赋予电池的外部,电池内外电位差的符号由电池内部相对于外部电位零值的电位确定。
In mathematical terms, the definition of voltage begins with the concept of an electric field E, a vector field assigning a magnitude and direction to each point in space. In many situations, the electric field is a conservative field, which means that it can be expressed as the gradient of a scalar function V, that is, E = –∇V. This scalar field V is referred to as the voltage distribution. Note that the definition allows for an arbitrary constant of integration—this is why absolute values of voltage are not meaningful. In general, electric fields can be treated as conservative only if magnetic fields do not significantly influence them, but this condition usually applies well to biological tissue.
用数学术语来说,电压的定义从电场的概念开始,电场 E, a是一个向量场,它为空间中的每一点分配一个大小和方向。在许多情况下,电场是一个保守场,这意味着它可以表示为一个标量函数的梯度, V, that is, E = –∇V. 即,。这个标量场称为电压分布。注意,这个定义允许任意的积分常数ーー这就是为什么电压的绝对值没有意义。一般来说,只有在磁场对电场影响不大的情况下,电场才能被认为是保守的,但这种情况通常适用于生物组织。
Because the electric field is the gradient of the voltage distribution, rapid changes in voltage within a small region imply a strong electric field; on the converse, if the voltage remains approximately the same over a large region, the electric fields in that region must be weak. A strong electric field, equivalent to a strong voltage gradient, implies that a strong force is exerted on any charged particles that lie within the region.
因为电场是电压分布的梯度,在一个小区域内电压的快速变化意味着一个强电场; 相反,如果在一个大区域内电压大致保持不变,那么该区域的电场必定是弱的。一个强电场,相当于一个强电压梯度,意味着一个强大的力施加在任何带电粒子的区域内。
Ions and the forces driving their motion
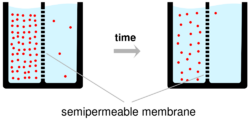
两个烧杯的原理图,每个烧杯装满水(浅蓝色)和一个半透膜,用一条虚线垂直插入烧杯,将烧杯中的液体分成相等的两部分。左手烧杯表示时间为零的初始状态,其中膜一侧的离子数(粉红色圆圈)远高于另一侧。右边的烧杯代表稍后时间点的情况,之后离子从烧杯的高浓度区间流过薄膜,使薄膜两侧的离子数目现在接近相等。
Electrical signals within biological organisms are, in general, driven by ions.[4] The most important cations for the action potential are sodium (Na+) and potassium (K+).[5] Both of these are monovalent cations that carry a single positive charge. Action potentials can also involve calcium (Ca2+),[6] which is a divalent cation that carries a double positive charge. The chloride anion (Cl−) plays a major role in the action potentials of some algae,[7] but plays a negligible role in the action potentials of most animals.[8]
一般来说,生物有机体内的电信号是由离子驱动的.[4] 。动作电位最重要的阳离子是钠(Na +)和钾(k +).[5] 。140–41.这两种阳离子都是单价阳离子,带有单个正电荷。动作电位也可以涉及钙(Ca2 +) ,[6] ,布洛克,Orkand 和格林内尔,pp。153–54.它是一种带有双正电荷的二价阳离子。氯离子(Cl -)在某些藻类的动作电位中起主要作用,[7],而在大多数动物的动作电位中起微不足道的作用.[8]。
Ions cross the cell membrane under two influences: diffusion and electric fields. A simple example wherein two solutions—A and B—are separated by a porous barrier illustrates that diffusion will ensure that they will eventually mix into equal solutions. This mixing occurs because of the difference in their concentrations. The region with high concentration will diffuse out toward the region with low concentration. To extend the example, let solution A have 30 sodium ions and 30 chloride ions. Also, let solution B have only 20 sodium ions and 20 chloride ions. Assuming the barrier allows both types of ions to travel through it, then a steady state will be reached whereby both solutions have 25 sodium ions and 25 chloride ions. If, however, the porous barrier is selective to which ions are let through, then diffusion alone will not determine the resulting solution. Returning to the previous example, let's now construct a barrier that is permeable only to sodium ions. Now, only sodium is allowed to diffuse cross the barrier from its higher concentration in solution A to the lower concentration in solution B. This will result in a greater accumulation of sodium ions than chloride ions in solution B and a lesser number of sodium ions than chloride ions in solution A.
离子在两种影响下穿过细胞膜: 扩散和电场。一个简单的例子,两个溶液ー a 和 b ー被一个多孔势垒隔开,说明扩散将确保它们最终混合成相等的溶液。这种混合是因为它们的浓度不同而发生的。高浓度区域向低浓度区域扩散。为了推广这个例子,让解决方案 a 有30个钠离子和30个氯离子。另外,让 b 溶液只含有20个钠离子和20个氯离子。假设势垒允许两种类型的离子通过它,那么两种溶液都将达到一个稳定状态,即都有25个钠离子和25个氯离子。然而,如果多孔势垒是选择性的让离子通过,那么扩散本身将不会决定最终的解决方案。回到前面的例子,让我们现在构造一个只能透过钠离子的势垒。现在,只有钠可以从溶液 a 中较高的浓度扩散到溶液 b 中较低的浓度。这将导致钠离子在 b 溶液中的积累大于氯离子,钠离子的数量少于在 a 溶液中的氯离子。
This means that there is a net positive charge in solution B from the higher concentration of positively charged sodium ions than negatively charged chloride ions. Likewise, there is a net negative charge in solution A from the greater concentration of negative chloride ions than positive sodium ions. Since opposite charges attract and like charges repel, the ions are now also influenced by electrical fields as well as forces of diffusion. Therefore, positive sodium ions will be less likely to travel to the now-more-positive B solution and remain in the now-more-negative A solution. The point at which the forces of the electric fields completely counteract the force due to diffusion is called the equilibrium potential. At this point, the net flow of the specific ion (in this case sodium) is zero.
这意味着在溶液 b 中存在一个净正电荷,这是由于带正电荷的钠离子比带负电荷的氯离子浓度高。同样,在溶液 a 中,由于负氯离子的浓度比正钠离子的浓度大,所以存在净负电荷。由于异性电荷相互吸引,同性电荷相互排斥,因此离子现在也受到电场和扩散力的影响。因此,正的钠离子将不太可能运行到现在更多的正的 b 解决方案,并留在现在更多的负的 a 解决方案。电场的力完全抵消扩散引起的力的那一点称为平衡势。此时,特定离子(在这种情况下是钠离子)的净流量为零。
Plasma membranes

Every cell is enclosed in a plasma membrane, which has the structure of a lipid bilayer with many types of large molecules embedded in it. Because it is made of lipid molecules, the plasma membrane intrinsically has a high electrical resistivity, in other words a low intrinsic permeability to ions. However, some of the molecules embedded in the membrane are capable either of actively transporting ions from one side of the membrane to the other or of providing channels through which they can move.[9]
每个细胞都被包裹在质膜中,质膜具有类脂双分子层的结构,其中包含许多类型的大分子。因为它是由类脂分子组成的,所以质膜本质上具有很高的电阻率,换句话说,对离子的内在渗透性很低。然而,嵌入在膜中的一些分子能够将离子从膜的一侧活跃地传送到另一侧,或者提供离子可以移动的通道.[9]。
In electrical terminology, the plasma membrane functions as a combined resistor and capacitor. Resistance arises from the fact that the membrane impedes the movement of charges across it. Capacitance arises from the fact that the lipid bilayer is so thin that an accumulation of charged particles on one side gives rise to an electrical force that pulls oppositely charged particles toward the other side. The capacitance of the membrane is relatively unaffected by the molecules that are embedded in it, so it has a more or less invariant value estimated at about 2 μF/cm2 (the total capacitance of a patch of membrane is proportional to its area). The conductance of a pure lipid bilayer is so low, on the other hand, that in biological situations it is always dominated by the conductance of alternative pathways provided by embedded molecules. Thus, the capacitance of the membrane is more or less fixed, but the resistance is highly variable.
在电学术语中,等离子膜起着电阻和电容的组合作用。电阻产生的原因是膜阻碍了电荷在膜上的运动。电容产生于这样一个事实,即脂双分子层是如此之薄,以至于一侧带电粒子的积累产生了一个电力,将带相反电荷的粒子拉向另一侧。膜的电容相对来说不受嵌入其中的分子的影响,因此它有一个或多或少的不变值,估计在2 μF/cm2左右(膜片的总电容与其面积成正比)。另一方面,纯类脂双分子层的电导率非常低,在生物学情况下,它总是由嵌入分子提供的替代通路的电导率决定。因此,膜的电容或多或少是固定的,但电阻是高度可变的。
The thickness of a plasma membrane is estimated to be about 7-8 nanometers. Because the membrane is so thin, it does not take a very large transmembrane voltage to create a strong electric field within it. Typical membrane potentials in animal cells are on the order of 100 millivolts (that is, one tenth of a volt), but calculations show that this generates an electric field close to the maximum that the membrane can sustain—it has been calculated that a voltage difference much larger than 200 millivolts could cause dielectric breakdown, that is, arcing across the membrane.
等离子体膜的厚度估计约为7-8纳米。因为膜非常薄,它不需要很大的跨膜电压就可以在其中产生强大的电场。动物细胞中典型的膜电位大约为100毫伏(即十分之一伏特) ,但计算表明,这种电位产生的电场接近膜所能承受的最大电场ー据计算,电压差大于200毫伏可能导致电介质击穿,也就是跨越膜的电弧。
Facilitated diffusion and transport
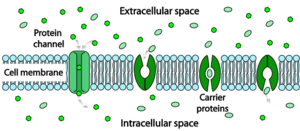
The resistance of a pure lipid bilayer to the passage of ions across it is very high, but structures embedded in the membrane can greatly enhance ion movement, either actively or passively, via mechanisms called facilitated transport and facilitated diffusion. The two types of structure that play the largest roles are ion channels and ion pumps, both usually formed from assemblages of protein molecules. Ion channels provide passageways through which ions can move. In most cases, an ion channel is permeable only to specific types of ions (for example, sodium and potassium but not chloride or calcium), and sometimes the permeability varies depending on the direction of ion movement. Ion pumps, also known as ion transporters or carrier proteins, actively transport specific types of ions from one side of the membrane to the other, sometimes using energy derived from metabolic processes to do so.
纯类脂双分子层对通过它的离子的抵抗力是非常高的,但是嵌入在膜中的结构可以极大地增强离子的运动,无论是主动的还是被动的,通过被称为促进转运和 facilitated diffusion 的机制。起主要作用的两种结构类型是离子通道和离子泵,它们通常都是由蛋白质分子组合而成。离子通道提供了离子可以移动的通道。在大多数情况下,离子通道只能通过特定类型的离子(例如,钠和钾,但不能通过氯化物或钙) ,有时通透性取决于离子运动的方向。离子泵,也被称为离子转运蛋白或载体蛋白,积极地将特定类型的离子从膜的一边运输到另一边,有时使用来自新陈代谢过程的能量来完成这项工作。
Ion pumps
Ion pumps are integral membrane proteins that carry out active transport, i.e., use cellular energy (ATP) to "pump" the ions against their concentration gradient.[10] Such ion pumps take in ions from one side of the membrane (decreasing its concentration there) and release them on the other side (increasing its concentration there).
离子泵是完整的膜蛋白,进行主动运输,也就是说,使用细胞能量(ATP)“泵”离子对他们的浓度梯度.[10]。这种离子泵从膜的一边吸收离子(降低那边的浓度) ,然后从另一边释放离子(增加那边的浓度)。
The ion pump most relevant to the action potential is the sodium–potassium pump, which transports three sodium ions out of the cell and two potassium ions in.[11] As a consequence, the concentration of potassium ions K+ inside the neuron is roughly 20-fold larger than the outside concentration, whereas the sodium concentration outside is roughly ninefold larger than inside.[12][13] In a similar manner, other ions have different concentrations inside and outside the neuron, such as calcium, chloride and magnesium.[13]
与动作电位最相关的离子泵是钠-钾离子泵,它将三个钠离子输出细胞,两个钾离子输入细胞.[11]。因此,钾离子在神经元内的浓度大约是外部浓度的20倍,而钠离子在神经元外的浓度大约是内部浓度的9倍.[12][13]。类似地,其他离子在神经元内外有不同的浓度,如钙、氯和镁.[13]。
If the numbers of each type of ion were equal, the sodium–potassium pump would be electrically neutral, but, because of the three-for-two exchange, it gives a net movement of one positive charge from intracellular to extracellular for each cycle, thereby contributing to a positive voltage difference. The pump has three effects: (1) it makes the sodium concentration high in the extracellular space and low in the intracellular space; (2) it makes the potassium concentration high in the intracellular space and low in the extracellular space; (3) it gives the intracellular space a negative voltage with respect to the extracellular space.
如果每种离子的数量相等,钠-钾离子泵就是电中性的,但是,由于这种三对二的交换,每个周期都会产生一个正电荷从细胞内向细胞外的净移动,从而产生正电压差。这种泵有三个作用: (1)它使细胞内的钠离子浓度高而细胞内的钠离子浓度低; (2)它使细胞内的钾离子浓度高而细胞内的钾离子浓度低; (3)它使细胞内的钾离子浓度高于细胞内的细胞外液细胞外液; (3)它使细胞内的钾离子浓度低于细胞内的细胞外液离子浓度。
The sodium-potassium pump is relatively slow in operation. If a cell were initialized with equal concentrations of sodium and potassium everywhere, it would take hours for the pump to establish equilibrium. The pump operates constantly, but becomes progressively less efficient as the concentrations of sodium and potassium available for pumping are reduced.
钠钾泵的工作速度较慢。如果一个细胞到处都是等浓度的钠和钾,那么泵需要几个小时才能达到平衡。这种泵经常运转,但随着可用于泵送的钠和钾浓度的降低,效率逐渐降低。
Ion pumps influence the action potential only by establishing the relative ratio of intracellular and extracellular ion concentrations. The action potential involves mainly the opening and closing of ion channels not ion pumps. If the ion pumps are turned off by removing their energy source, or by adding an inhibitor such as ouabain, the axon can still fire hundreds of thousands of action potentials before their amplitudes begin to decay significantly.[10] In particular, ion pumps play no significant role in the repolarization of the membrane after an action potential.[5]
离子泵仅通过建立细胞内和细胞外离子浓度的相对比例来影响动作电位。动作电位主要涉及离子通道的开闭,而非离子泵。如果通过移除离子泵的能量源或者加入 ouabain 这样的抑制剂来关闭离子泵,轴突仍然可以在其振幅开始明显衰减之前激发数十万个动作电位.[10] 。特别是,离子泵在动作电位后细胞膜的复极化过程中没有发挥重要作用.[5]。
Another functionally important ion pump is the sodium-calcium exchanger. This pump operates in a conceptually similar way to the sodium-potassium pump, except that in each cycle it exchanges three Na+ from the extracellular space for one Ca++ from the intracellular space. Because the net flow of charge is inward, this pump runs "downhill", in effect, and therefore does not require any energy source except the membrane voltage. Its most important effect is to pump calcium outward—it also allows an inward flow of sodium, thereby counteracting the sodium-potassium pump, but, because overall sodium and potassium concentrations are much higher than calcium concentrations, this effect is relatively unimportant. The net result of the sodium-calcium exchanger is that in the resting state, intracellular calcium concentrations become very low.
另一个重要的功能离子泵是钠钙交换器。这种泵的工作原理与钠-钾泵相似,只是在每个循环中,它从细胞内空间从细胞外液中交换3个钠离子来换取1个钙离子。因为电荷净流量是向内的,这个泵实际上是“下坡”的,因此除了膜电位之外不需要任何能源。它最重要的作用是将钙向外泵出ーー它还允许钠向内流动,从而抵消了钠钾泵,但是,由于总的钠钾浓度远高于钙浓度,这种作用相对来说并不重要。钠钙交换的最终结果是在静息状态下,细胞内钙浓度变得非常低。
Ion channels
Ion channels are integral membrane proteins with a pore through which ions can travel between extracellular space and cell interior. Most channels are specific (selective) for one ion; for example, most potassium channels are characterized by 1000:1 selectivity ratio for potassium over sodium, though potassium and sodium ions have the same charge and differ only slightly in their radius. The channel pore is typically so small that ions must pass through it in single-file order.[15] Channel pores can be either open or closed for ion passage, although a number of channels demonstrate various sub-conductance levels. When a channel is open, ions permeate through the channel pore down the transmembrane concentration gradient for that particular ion. Rate of ionic flow through the channel, i.e. single-channel current amplitude, is determined by the maximum channel conductance and electrochemical driving force for that ion, which is the difference between the instantaneous value of the membrane potential and the value of the reversal potential.[16]
离子通道是一种完整的膜蛋白,它有一个孔,离子可以通过这个孔在细胞外液和细胞内部之间穿梭。大多数钾离子通道对单个离子具有特异性(选择性) ; 例如,大多数钾离子通道对钾的选择性比为1000:1,而钾离子和钠离子的拥有属性相同,只是半径略有不同。通道孔通常非常小,以至于离子必须以单列顺序通过.[15]。虽然一些通道表现出不同的次电导水平,但是通道孔可以为离子通过而打开或关闭。当通道打开时,离子通过通道孔,沿着该特定离子的跨膜浓度梯度向下渗透。离子通过通道的速率,即。单通道电流的幅度,是由该离子的最大通道电导和电化学驱动力决定的,即膜电位的瞬时值与翻转电位值之间的差值.[16]。朱格,页。33–37.
Schematic stick diagram of a tetrameric potassium channel where each of the monomeric subunits is symmetrically arranged around a central ion conduction pore. The pore axis is displayed perpendicular to the screen. Carbon, oxygen, and nitrogen atom are represented by grey, red, and blue spheres, respectively. A single potassium cation is depicted as a purple sphere in the center of the channel.
四聚体钾离子通道的简图,其中每个单体亚基都对称地排列在中央离子导电孔周围。孔轴与屏幕垂直显示。碳原子、氧原子和氮原子分别用灰色、红色和蓝色球体表示。一个单独的钾离子被描绘成通道中心的一个紫色球体。
A channel may have several different states (corresponding to different conformations of the protein), but each such state is either open or closed. In general, closed states correspond either to a contraction of the pore—making it impassable to the ion—or to a separate part of the protein, stoppering the pore. For example, the voltage-dependent sodium channel undergoes inactivation, in which a portion of the protein swings into the pore, sealing it.[17] This inactivation shuts off the sodium current and plays a critical role in the action potential.
一个通道可能有几种不同的状态(对应于蛋白质的不同构象) ,但每种状态要么是开放的,要么是关闭的。一般来说,闭合状态要么对应于孔的收缩ーー使其不能通过离子ーー要么对应于蛋白质的一个单独部分,堵塞孔。例如,依赖电压的钠通道失活,其中一部分蛋白质摆动进入孔隙,封闭它。这种失活切断了钠电流,在动作电位中起着关键作用。
Ion channels can be classified by how they respond to their environment.[18] For example, the ion channels involved in the action potential are voltage-sensitive channels; they open and close in response to the voltage across the membrane. Ligand-gated channels form another important class; these ion channels open and close in response to the binding of a ligand molecule, such as a neurotransmitter. Other ion channels open and close with mechanical forces. Still other ion channels—such as those of sensory neurons—open and close in response to other stimuli, such as light, temperature or pressure.
离子通道可以根据它们对环境的反应来分类.[18] 。例如,与动作电位有关的离子通道是电压敏感通道,它们随着跨膜电压的变化而开闭。配体门控通道形成另一个重要类别,这些离子通道开放和关闭响应配体分子的结合,如神经递质。其他离子通道的开启和关闭都受到机械力的作用。还有一些离子通道(如感觉神经元的通道)在其他刺激(如光、温度或压力)的作用下开关。
Leakage channels
Leakage channels are the simplest type of ion channel, in that their permeability is more or less constant. The types of leakage channels that have the greatest significance in neurons are potassium and chloride channels. Even these are not perfectly constant in their properties: First, most of them are voltage-dependent in the sense that they conduct better in one direction than the other (in other words, they are rectifiers); second, some of them are capable of being shut off by chemical ligands even though they do not require ligands in order to operate.
泄漏通道是最简单的离子通道类型,因为它们的渗透率几乎是恒定的。在神经元中,钾离子通道和氯离子通道是泄漏通道中最重要的类型。即使它们的性质也不是完全恒定的: 首先,它们中的大多数是电压依赖性的,因为它们在一个方向上比在另一个方向上导电更好(换句话说,它们是整流器) ; 其次,它们中的一些能够被化学配体关闭,即使它们不需要配体来操作。
Ligand-gated channels
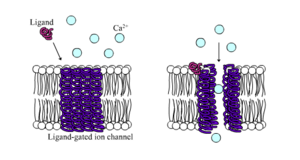
Ligand-gated ion channels are channels whose permeability is greatly increased when some type of chemical ligand binds to the protein structure. Animal cells contain hundreds, if not thousands, of types of these. A large subset function as neurotransmitter receptors—they occur at postsynaptic sites, and the chemical ligand that gates them is released by the presynaptic axon terminal. One example of this type is the AMPA receptor, a receptor for the neurotransmitter glutamate that when activated allows passage of sodium and potassium ions. Another example is the GABAA receptor, a receptor for the neurotransmitter GABA that when activated allows passage of chloride ions.
配体门控离子通道是当某种类型的化学配体与蛋白质结构结合时,其通透性大大增加的通道。动物细胞包含成百上千种这样的细胞。神经递质受体的一个很大的子集功能ーー它们发生在突触后位点,而与它们相关的化学配体是由突触前轴突末端释放的。这种类型的一个例子是 AMPA 受体,一种神经递质谷氨酸的受体,当激活时允许钠离子和钾离子通过。另一个例子是 GABA < sub > a 受体,一种神经递质 GABA 的受体,当被激活时允许氯离子通过。
Neurotransmitter receptors are activated by ligands that appear in the extracellular area, but there are other types of ligand-gated channels that are controlled by interactions on the intracellular side.
神经递质受体被出现在细胞外区的配体激活,但是还有其他类型的配体门控通道是由细胞内的相互作用控制的。
Voltage-dependent channels
Voltage-gated ion channels, also known as voltage dependent ion channels, are channels whose permeability is influenced by the membrane potential. They form another very large group, with each member having a particular ion selectivity and a particular voltage dependence. Many are also time-dependent—in other words, they do not respond immediately to a voltage change but only after a delay.
电压依赖性通道,也称为电压依赖性离子通道,是一种通道,其通透性受膜电位的影响。它们形成了另一个非常大的基团,每个成员具有特定的离子选择性和特定的电压依赖性。其中许多还与时间有关ー换句话说,它们不会立即对电压变化作出反应,而只是在延迟之后才作出反应。
One of the most important members of this group is a type of voltage-gated sodium channel that underlies action potentials—these are sometimes called Hodgkin-Huxley sodium channels because they were initially characterized by Alan Lloyd Hodgkin and Andrew Huxley in their Nobel Prize-winning studies of the physiology of the action potential. The channel is closed at the resting voltage level, but opens abruptly when the voltage exceeds a certain threshold, allowing a large influx of sodium ions that produces a very rapid change in the membrane potential. Recovery from an action potential is partly dependent on a type of voltage-gated potassium channel that is closed at the resting voltage level but opens as a consequence of the large voltage change produced during the action potential.
这个小组最重要的成员之一是一种作为动作电位基础的电压门控钠通道ーー这些通道有时被称为 Hodgkin-Huxley 钠通道,因为在他们获得诺贝尔奖的动作电位生理学研究中,他们最初是拥有属性艾伦·劳埃德·霍奇金和 Andrew Huxley。通道在静息电压水平处关闭,但当电压超过一定阈值时突然打开,从而允许大量钠离子流入,使膜电位发生非常迅速的变化。从动作电位中恢复部分依赖于一种在静息电压水平关闭但在动作电位产生巨大电压变化时打开的电压门控钾离子通道。
Reversal potential
The reversal potential (or equilibrium potential) of an ion is the value of transmembrane voltage at which diffusive and electrical forces counterbalance, so that there is no net ion flow across the membrane. This means that the transmembrane voltage exactly opposes the force of diffusion of the ion, such that the net current of the ion across the membrane is zero and unchanging. The reversal potential is important because it gives the voltage that acts on channels permeable to that ion—in other words, it gives the voltage that the ion concentration gradient generates when it acts as a battery.
一个离子的翻转电位电位(或平衡电位)是一个跨膜电压的值,在这个电压下扩散力和电力相互抵消,因此没有净离子流过这个跨膜电位。这意味着跨膜电压完全对抗离子的扩散力,使得跨膜离子的净电流为零且不变。翻转电位是重要的,因为它提供了作用于离子可渗透通道的电压---- 换句话说,它提供了离子浓度梯度作为电池时产生的电压。
The equilibrium potential of a particular ion is usually designated by the notation Eion.The equilibrium potential for any ion can be calculated using the Nernst equation.[19] For example, reversal potential for potassium ions will be as follows:
一个特定离子的平衡电位通常用记号 Eion 来表示。任何离子的平衡电位都可以用能斯特方程来计算.[19] 。普尔维斯等人。28-32; 布洛克,Orkand 和格林内尔,pp。133–134; Schmidt-Nielsen, pp.478-480,596-597; Junge,pp.33-35例如,钾离子的翻转电位如下:
- [math]\displaystyle{ E_{eq,K^+} = \frac{RT}{zF} \ln \frac{[K^+]_{o}}{[K^+]_{i}} , }[/math]
- E_{eq,K^+} = \frac{RT}{zF} \ln \frac{[K^+]_{o}}{[K^+]_{i}} ,
- e _ { eq,k ^ + } = frac { RT }{ zF } ln frac {[ k ^ + ]{ o }{[ k ^ + ]{ i } ,
where
- Eeq,K+ is the equilibrium potential for potassium, measured in volts
- R is the universal gas constant, equal to 8.314 joules·K−1·mol−1
- T is the absolute temperature, measured in kelvins (= K = degrees Celsius + 273.15)
- z is the number of elementary charges of the ion in question involved in the reaction
- F is the Faraday constant, equal to 96,485 coulombs·mol−1 or J·V−1·mol−1
- [K+]o is the extracellular concentration of potassium, measured in mol·m−3 or mmol·l−1
- [K+]i is the intracellular concentration of potassium
其中
- Eeq,K+ 是钾的平衡电位,用伏特为单位
- R 为通用气体常数,等于 8.314 joules·K−1·mol−1
- T 为绝对温度,以 K 为单位 in kelvins (= K = degrees Celsius + 273.15)
- z 反应中所涉及的离子的基本电荷数
- F 是法拉第常数,等于 96,485 coulombs·mol−1 is the Faraday constant, equal to 96,485 coulombs·mol−1 or J·V−1·mol−1
- [K+]o 为钾离子的胞外浓度 measured in mol·m−3 or mmol·l−1
- [K+]i is 为钾离子的胞内浓度,
即使两种离子具有相同的电荷(即 K+ and Na+ ),只要胞内外浓度不同,它们仍然具有非常不同的平衡电位。以神经元中钾和钠的平衡电位为例,钾离子在胞内为 140 mM,胞外 5 mM,平衡电位为 -84 mV;而钠离子胞内约 12 mM,胞外 140 mM,平衡电位 ENa 约为 +66 mV [note 1]。
发育中膜电位的变化
A neuron's resting membrane potential actually changes during the development of an organism. In order for a neuron to eventually adopt its full adult function, its potential must be tightly regulated during development. As an organism progresses through development the resting membrane potential becomes more negative.[20] Glial cells are also differentiating and proliferating as development progresses in the brain.[21] The addition of these glial cells increases the organism's ability to regulate extracellular potassium. The drop in extracellular potassium can lead to a decrease in membrane potential of 35 mV.[22]
神经元的静息膜电位在生物体发育过程中会发生变化。为了让神经元最终发挥其完整的成年期功能,它的潜能必须在发育过程中受到严格的调控。随着生物体的发育,静息膜电位变得更负 [20] 。随着脑的发育,神经胶质细胞也在分化和增殖 [21]。增加的神经胶质细胞增加了机体调节细胞外钾的能力。细胞外液中的钾的下降可导致膜电位下降 35 mV [22]。
细胞兴奋性
细胞兴奋性(Cell excitability)是各种组织中的细胞反应所必需的细胞膜电位变化。细胞兴奋性是早期胚胎发生过程中诱导的一种特性 [23]。细胞兴奋性也被定义为引起反应的容易程度 [24] 。静息电位和阈电位是细胞兴奋性的基础,这些过程是细胞剂量电位和动作电位产生的基础。
细胞兴奋性最重要的调节因子是细胞外电解质浓度(即 Na+, K+, Ca2+, Cl−, Mg2+)及其相关蛋白。调节细胞兴奋性的重要蛋白质是电压门控离子通道、离子转运蛋白(如钠钾 ATP 酶、镁转运蛋白、酸碱转运蛋白)、膜受体和超极化激活的环核苷酸门控通道 [25]。例如,钾离子通道和钙敏感受体是神经元、心肌细胞和星形胶质细胞等其他兴奋性细胞的兴奋性的重要调节因子[26] 。钙离子也是可兴奋细胞信号转导中最重要的第二信使。突触受体的激活产生神经元兴奋性的长期改变 [27]。甲状腺激素、肾上腺激素和其他激素也调节细胞的兴奋性,例如,孕酮和雌激素调节子宫平滑肌细胞的兴奋性。
Many cell types are considered to have an excitable membrane. Excitable cells are neurons, myocytes (cardiac, skeletal, smooth), vascular endothelial cells, pericytes, juxtaglomerular cells, interstitial cells of Cajal, many types of epithelial cells (e.g. beta cells, alpha cells, delta cells, enteroendocrine cells, pulmonary neuroendocrine cells, pinealocytes), glial cells (e.g. astrocytes), mechanoreceptor cells (e.g. hair cells and Merkel cells), chemoreceptor cells (e.g. glomus cells, taste receptors), some plant cells and possibly immune cells.[28] Astrocytes display a form of non-electrical excitability based on intracellular calcium variations related to the expression of several receptors through which they can detect the synaptic signal. In neurons, there are different membrane properties in some portions of the cell, for example, dendritic excitability endows neurons with the capacity for coincidence detection of spatially separated inputs.[29]
许多细胞类型被认为具有兴奋性膜。兴奋性细胞包括神经元、肌细胞(心肌细胞、骨骼肌细胞、平滑肌细胞)、血管内皮细胞、周细胞、肾小球旁细胞、Cajal 间质细胞、多种类型的上皮细胞(如 β 细胞、α 细胞、δ 细胞、肠内分泌细胞、肺神经内分泌细胞、松果体细胞)、胶质细胞(例如星形胶质细胞)、机械感觉受体细胞(例如毛细胞和 Merkel 细胞)、化学感觉受体细胞(例如血管球细胞、味觉受体细胞)、一些植物细胞,可能还有免疫细胞 [28]。星形胶质细胞表现出一种非电兴奋性,这种兴奋性是基于细胞内钙离子的变化,这种变化与几个受体的表达有关,通过这些受体它们可以检测到突触信号。在神经元中,细胞的某些部分具有不同的膜特性,例如,树突的兴奋性赋予神经元对空间上分离的输入信号进行重合检测的能力 [29]。
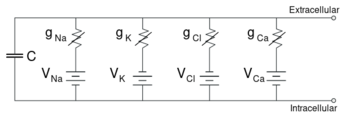
Equivalent circuit等效电路
Electrophysiologists model the effects of ionic concentration differences, ion channels, and membrane capacitance in terms of an equivalent circuit, which is intended to represent the electrical properties of a small patch of membrane. The equivalent circuit consists of a capacitor in parallel with four pathways each consisting of a battery in series with a variable conductance. The capacitance is determined by the properties of the lipid bilayer, and is taken to be fixed. Each of the four parallel pathways comes from one of the principal ions, sodium, potassium, chloride, and calcium. The voltage of each ionic pathway is determined by the concentrations of the ion on each side of the membrane; see the Reversal potential section above. The conductance of each ionic pathway at any point in time is determined by the states of all the ion channels that are potentially permeable to that ion, including leakage channels, ligand-gated channels, and voltage-gated ion channels.
电生理学家用等效电路模拟离子浓度差、离子通道和膜电容的影响,用以表示一小块膜片的电性能。该等效电路由一个并联电容器和四条通路组成,每条通路由一个可变电导的串联电池组成。电容是由类脂双分子层的性质决定的,并被认为是固定的。四条平行通路中的每一条都来自于一种主要离子,钠、钾、氯和钙。每个离子通路的电压由膜两侧的离子浓度决定; 见上面的翻转电位。每个离子通道在任何时间点的电导都是由所有离子通道的状态决定的,这些离子通道对该离子具有潜在的渗透性,包括泄漏通道、配体门控通道和电压门控离子通道。
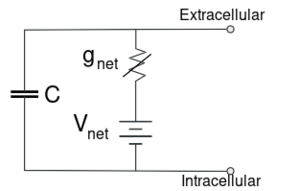
For fixed ion concentrations and fixed values of ion channel conductance, the equivalent circuit can be further reduced, using the Goldman equation as described below, to a circuit containing a capacitance in parallel with a battery and conductance. In electrical terms, this is a type of RC circuit (resistance-capacitance circuit), and its electrical properties are very simple. Starting from any initial state, the current flowing across either the conductance or the capacitance decays with an exponential time course, with a time constant of τ = RC, where C is the capacitance of the membrane patch, and R = 1/gnet is the net resistance. For realistic situations, the time constant usually lies in the 1—100 millisecond range. In most cases, changes in the conductance of ion channels occur on a faster time scale, so an RC circuit is not a good approximation; however, the differential equation used to model a membrane patch is commonly a modified version of the RC circuit equation.
对于固定的离子浓度和固定的离子通道电导值,等效电路可以进一步缩小,使用下面描述的戈德曼方程电路,变成一个包含电容和电池电导并联的电路。在电学术语中,这是一种 RC 电路(阻容电路) ,其电学特性非常简单。从任何初始状态开始,流过电导或电容的电流以 EXPTIME 衰变,其时间常数为,这里是膜片的电容,这里是网电阻。在现实情况下,时间常数一般在1ー100毫秒的范围内。在大多数情况下,离子通道电导的变化发生在一个更快的时间尺度上,所以 RC 电路不是一个好的近似值; 然而,用于模拟膜片的微分方程通常是 RC 电路方程的修正版。
Resting potential
When the membrane potential of a cell goes for a long period of time without changing significantly, it is referred to as a resting potential or resting voltage. This term is used for the membrane potential of non-excitable cells, but also for the membrane potential of excitable cells in the absence of excitation. In excitable cells, the other possible states are graded membrane potentials (of variable amplitude), and action potentials, which are large, all-or-nothing rises in membrane potential that usually follow a fixed time course. Excitable cells include neurons, muscle cells, and some secretory cells in glands. Even in other types of cells, however, the membrane voltage can undergo changes in response to environmental or intracellular stimuli. For example, depolarization of the plasma membrane appears to be an important step in programmed cell death.[30]
= = 静息电位 = = 当一个细胞的膜电位长时间不发生明显变化时,它被称为静息电位电压或静息电压。这个术语用于描述不可兴奋细胞的膜电位,也用于描述缺乏兴奋时可兴奋细胞的膜电位。在可兴奋细胞中,其他可能的状态是分级膜电位(可变振幅)和动作电位,这些电位通常在一个固定的时间过程中,在膜电位一分钟内上升很大,要么全有要么全无。可兴奋细胞包括神经元、肌细胞和腺体中的一些分泌细胞。然而,即使在其他类型的细胞中,膜电位也会因为环境或细胞内的刺激而发生变化。例如,去极化的质膜似乎是一个重要的步骤,在细胞程序性死亡.[30]。
The interactions that generate the resting potential are modeled by the Goldman equation.[31] This is similar in form to the Nernst equation shown above, in that it is based on the charges of the ions in question, as well as the difference between their inside and outside concentrations. However, it also takes into consideration the relative permeability of the plasma membrane to each ion in question.
产生静息电位的相互作用是由戈德曼方程研究所模拟的.[31] 。32-33; 布洛克,Orkand,格林内尔,pp。138–140; Schmidt-Nielsen, pp.480; Junge,pp.35-37这在形式上类似于上面所示的能斯特方程,因为它是根据有关离子的电荷以及它们内外浓度之间的差别而建立的。然而,它也考虑到质膜对每个离子的相对渗透性。
- [math]\displaystyle{ E_{m} = \frac{RT}{F} \ln{ \left( \frac{ P_{\mathrm{K}}[\mathrm{K}^{+}]_\mathrm{out} + P_{\mathrm{Na}}[\mathrm{Na}^{+}]_\mathrm{out} + P_{\mathrm{Cl}}[\mathrm{Cl}^{-}]_\mathrm{in}}{ P_{\mathrm{K}}[\mathrm{K}^{+}]_\mathrm{in} + P_{\mathrm{Na}}[\mathrm{Na}^{+}]_\mathrm{in} + P_{\mathrm{Cl}}[\mathrm{Cl}^{-}]_\mathrm{out}} \right) } }[/math]
E_{m} = \frac{RT}{F} \ln{ \left( \frac{ P_{\mathrm{K}}[\mathrm{K}^{+}]_\mathrm{out} + P_{\mathrm{Na}}[\mathrm{Na}^{+}]_\mathrm{out} + P_{\mathrm{Cl}}[\mathrm{Cl}^{-}]_\mathrm{in}}{ P_{\mathrm{K}}[\mathrm{K}^{+}]_\mathrm{in} + P_{\mathrm{Na}}[\mathrm{Na}^{+}]_\mathrm{in} + P_{\mathrm{Cl}}[\mathrm{Cl}^{-}]_\mathrm{out}} \right) }
句子太长,请提供一个短句
The three ions that appear in this equation are potassium (K+), sodium (Na+), and chloride (Cl−). Calcium is omitted, but can be added to deal with situations in which it plays a significant role.[32] Being an anion, the chloride terms are treated differently from the cation terms; the intracellular concentration is in the numerator, and the extracellular concentration in the denominator, which is reversed from the cation terms. Pi stands for the relative permeability of the ion type i.
在这个方程式中出现的三个离子是钾(k +)、钠(Na +)和氯(Cl -)。钙是省略的,但可以添加到处理的情况下,它发挥了重要的作用le.[32] 。作为一个阴离子,氯离子项处理不同于阳离子项; 细胞内浓度是在分子,胞外浓度在分母,这是反向阳离子项。Pi 代表离子类型 i 的相对渗透率。
In essence, the Goldman formula expresses the membrane potential as a weighted average of the reversal potentials for the individual ion types, weighted by permeability. (Although the membrane potential changes about 100 mV during an action potential, the concentrations of ions inside and outside the cell do not change significantly. They remain close to their respective concentrations when then membrane is at resting potential.) In most animal cells, the permeability to potassium is much higher in the resting state than the permeability to sodium. As a consequence, the resting potential is usually close to the potassium reversal potential.[33][34] The permeability to chloride can be high enough to be significant, but, unlike the other ions, chloride is not actively pumped, and therefore equilibrates at a reversal potential very close to the resting potential determined by the other ions.
从本质上讲,高盛公式将膜电位表示为单个离子类型的逆转势的加权平均数,通过渗透率加权。(虽然膜电位在动作电位期间会发生100mv 左右的变化,但细胞内外的离子浓度不会发生显著变化。当膜处于静息电位时,它们仍然接近各自的浓度。)在大多数动物细胞中,静息状态下钾的通透性比钠的通透性高得多。As a consequence, the resting potential is usually close to the potassium reversal potential.Purves et al., p. 34; Bullock, Orkand, and Grinnell, p. 134; Schmidt-Nielsen, pp.478-480. Purves et al. ,pp.33-36; 布洛克,Orkand 和格林内尔,p. 131。但是,与其他离子不同的是,氯离子没有被主动泵入,因此平衡的翻转电位非常接近由其他离子决定的静息电位。l.[33][34]
Values of resting membrane potential in most animal cells usually vary between the potassium reversal potential (usually around -80 mV) and around -40 mV. The resting potential in excitable cells (capable of producing action potentials) is usually near -60 mV—more depolarized voltages would lead to spontaneous generation of action potentials. Immature or undifferentiated cells show highly variable values of resting voltage, usually significantly more positive than in differentiated cells.[35] In such cells, the resting potential value correlates with the degree of differentiation: undifferentiated cells in some cases may not show any transmembrane voltage difference at all.
在大多数动物细胞中,静息膜电位的数值通常在翻转电位(通常在 -80 mV)和 -40 mV 之间变化。可兴奋细胞(能够产生动作电位)的静息电位通常接近60mv ー更多的去极化电压会导致动作电位的自然发生。未成熟或未分化细胞的静息电压变化很大,通常明显高于已分化的细胞.[35] 。在这些细胞中,静息电位值与分化程度相关: 在某些情况下未分化的细胞可能根本没有任何跨膜电压差。
Maintenance of the resting potential can be metabolically costly for a cell because of its requirement for active pumping of ions to counteract losses due to leakage channels. The cost is highest when the cell function requires an especially depolarized value of membrane voltage. For example, the resting potential in daylight-adapted blowfly (Calliphora vicina) photoreceptors can be as high as -30 mV.[36] This elevated membrane potential allows the cells to respond very rapidly to visual inputs; the cost is that maintenance of the resting potential may consume more than 20% of overall cellular ATP.[37]
对于电池来说,维护静息电位的代谢成本可能很高,因为它需要主动泵入离子来抵消泄漏通道造成的损失。当细胞功能需要特别去极化值膜电位时,成本最高。例如,静息电位在日光适应的绿头丽蝇(红头丽蝇)光感受器可高达 -30mv.[36] 。这种升高的膜电位可以使细胞对视觉输入作出非常迅速的反应; 维持静息电位可能消耗超过20% 的细胞总 ATP.[37]。
On the other hand, the high resting potential in undifferentiated cells does not necessarily incur a high metabolic cost. This apparent paradox is resolved by examination of the origin of that resting potential. Little-differentiated cells are characterized by extremely high input resistance,[35] which implies that few leakage channels are present at this stage of cell life. As an apparent result, potassium permeability becomes similar to that for sodium ions, which places resting potential in-between the reversal potentials for sodium and potassium as discussed above. The reduced leakage currents also mean there is little need for active pumping in order to compensate, therefore low metabolic cost.
另一方面,未分化细胞中的高静息电位并不一定导致高代谢成本。这个明显的悖论通过研究静息电位的起源得到了解决。小分化细胞具有极高的拥有属性输入电阻,[35] ,这意味着在细胞生命的这个阶段很少有泄漏通道存在。作为一个明显的结果,钾离子的渗透性变得类似于钠离子的渗透性,正如上面讨论的,钠离子和钾离子的反转电位之间有静息电位。泄漏电流的减少也意味着不需要主动抽水来补偿,因此代谢成本低。
Graded potentials
As explained above, the potential at any point in a cell's membrane is determined by the ion concentration differences between the intracellular and extracellular areas, and by the permeability of the membrane to each type of ion. The ion concentrations do not normally change very quickly (with the exception of Ca2+, where the baseline intracellular concentration is so low that even a small influx may increase it by orders of magnitude), but the permeabilities of the ions can change in a fraction of a millisecond, as a result of activation of ligand-gated ion channels. The change in membrane potential can be either large or small, depending on how many ion channels are activated and what type they are, and can be either long or short, depending on the lengths of time that the channels remain open. Changes of this type are referred to as graded potentials, in contrast to action potentials, which have a fixed amplitude and time course.
= = 分级电位 = = 如上所述,细胞膜上任何一点的电位取决于细胞内和细胞外区域离子浓度的差异,以及细胞膜对每种离子的通透性。离子浓度通常不会很快改变(除了 Ca2 + ,其细胞内基线浓度非常低,即使是很小的内流量也可能增加1毫秒数量级) ,但是由于配体门控离子通道的激活,离子的通透性可以在几分之一毫秒内改变。膜电位的变化可大可小,取决于激活的离子通道的数量和类型,也可长可短,取决于通道保持开放的时间长短。这种类型的变化被称为分级电位,与动作电位相反,动作电位有固定的振幅和时间进程。
As can be derived from the Goldman equation shown above, the effect of increasing the permeability of a membrane to a particular type of ion shifts the membrane potential toward the reversal potential for that ion. Thus, opening Na+ channels shifts the membrane potential toward the Na+ reversal potential, which is usually around +100 mV. Likewise, opening K+ channels shifts the membrane potential toward about –90 mV, and opening Cl− channels shifts it toward about –70 mV (resting potential of most membranes). Thus, Na+ channels shift the membrane potential in a positive direction, K+ channels shift it in a negative direction (except when the membrane is hyperpolarized to a value more negative than the K+ reversal potential), and Cl− channels tend to shift it towards the resting potential.
正如我们从上面的戈德曼方程中得出的结论,增加膜的渗透性对于特定类型离子的影响会使膜电位向翻转电位的方向移动。因此,开放的钠离子通道将膜电位向钠离子翻转电位转移,通常在 + 100 mV 左右。同样,开放 k + 通道使膜电位向大约 -90 mV 的方向移动,开放 Cl 通道使其向大约 -70 mV 的方向移动(大多数膜的静息电位)。因此,Na + 通道使膜电位向正方向移动,k + 通道使其向负方向移动(除非膜超极化到比 k + 翻转电位更负的程度) ,而 Cl-通道则倾向于使其向静息电位方向移动。
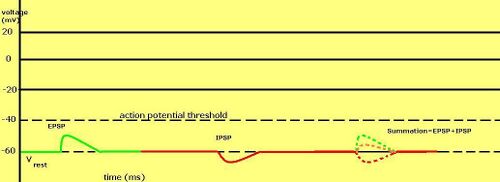
Graded membrane potentials are particularly important in neurons, where they are produced by synapses—a temporary change in membrane potential produced by activation of a synapse by a single graded or action potential is called a postsynaptic potential. Neurotransmitters that act to open Na+ channels typically cause the membrane potential to become more positive, while neurotransmitters that activate K+ channels typically cause it to become more negative; those that inhibit these channels tend to have the opposite effect.
分级膜电位的总和在神经元中尤其重要,因为它们是由突触产生的---- 由单个分级或动作电位激活突触而产生的突触膜电位的暂时变化称为突触后电位。神经递质的作用是打开 Na + 通道,典型地导致膜电位变得更加积极,而神经递质的作用是激活 k + 通道,典型地导致它变得更加消极; 抑制这些通道的神经递质往往有相反的效果。
Whether a postsynaptic potential is considered excitatory or inhibitory depends on the reversal potential for the ions of that current, and the threshold for the cell to fire an action potential (around –50mV). A postsynaptic current with a reversal potential above threshold, such as a typical Na+ current, is considered excitatory. A current with a reversal potential below threshold, such as a typical K+ current, is considered inhibitory. A current with a reversal potential above the resting potential, but below threshold, will not by itself elicit action potentials, but will produce subthreshold membrane potential oscillations. Thus, neurotransmitters that act to open Na+ channels produce excitatory postsynaptic potentials, or EPSPs, whereas neurotransmitters that act to open K+ or Cl− channels typically produce inhibitory postsynaptic potentials, or IPSPs. When multiple types of channels are open within the same time period, their postsynaptic potentials summate (are added together).
突触后电位是兴奋性电位还是抑制性电位取决于该电流离子的翻转电位,以及细胞激发动作电位的阈值(大约-50mV)。如果突触后电流超过阈值,如典型的钠离子电流,则被认为是兴奋性的。翻转电位。低于阈值的翻转电位,如典型的 k + 电流,被认为是抑制电流。一个翻转电位高于静息电位阈值但低于阈值的电流本身不会引起动作电位,但是会产生阈值以下的膜电位振荡。因此,作用于开放 Na + 通道的神经递质产生兴奋性突触后电位(epsp) ,而作用于开放 k + 或 Cl-通道的神经递质通常产生抑制性突触后电位(ipsp)。当多种类型的通道在同一时间段内开放时,它们的突触后电位相加。
Other values 其他值
从生物物理学的观点来看,静息膜电位不过是细胞未受刺激时主导的细胞膜通透性所产生的膜电位。上面的加权平均方程总是适用的,但是下面的方法可能更容易可视化。在任何给定的时刻,离子有两个因素决定其对细胞膜电位的影响:
- 离子的驱动力
- 离子的渗透性
如果驱动力很大,那么离子就会被“推”过膜。如果渗透性很高,离子就更容易跨膜扩散。
- Driving force is the net electrical force available to move that ion across the membrane. It is calculated as the difference between the voltage that the ion "wants" to be at (its equilibrium potential) and the actual membrane potential (Em). So, in formal terms, the driving force for an ion = Em - Eion
- For example, at our earlier calculated resting potential of −73 mV, the driving force on potassium is 7 mV : (−73 mV) − (−80 mV) = 7 mV. The driving force on sodium would be (−73 mV) − (60 mV) = −133 mV.
- Permeability is a measure of how easily an ion can cross the membrane. It is normally measured as the (electrical) conductance and the unit, siemens, corresponds to 1 C·s−1·V−1, that is one coulomb per second per volt of potential.
- 驱动力是使离子在膜上移动的净电力。它被计算为离子“希望”处于的电压(其平衡电位)和实际膜电位(Em)之间的差值。因此,在正式术语中,离子的驱动力 = Em-Eion
- 例如,在我们早先计算的静息电位为-73 mV,钾的驱动力为7 mV: (- 73 mV)-(- 80 mV) = 7 mV。钠离子的驱动力为(- 73 mV)-(60 mV) =-133 mV。
- 渗透性是衡量离子通过细胞膜的容易程度。它通常被测量为(电)导和单位,西门子,相当于1 c s-1 v-1,即一库仑每秒每伏特的电位。
So, in a resting membrane, while the driving force for potassium is low, its permeability is very high. Sodium has a huge driving force but almost no resting permeability. In this case, potassium carries about 20 times more current than sodium, and thus has 20 times more influence over Em than does sodium.
因此,在静息膜中,钾离子的驱动力较低,而钾离子的通透性却很高。钠具有巨大的驱动力,但几乎没有静息通透性。在这种情况下,钾的电流是钠的20倍,因此对 Em 的影响是钠的20倍。
However, consider another case—the peak of the action potential. Here, permeability to Na is high and K permeability is relatively low. Thus, the membrane moves to near ENa and far from EK.
然而,考虑另一种情况ーー动作电位的峰值。在这里,钠的渗透率较高,钾的渗透率相对较低。因此,膜移动到近 ENa 和远离 EK。
The more ions are permeant the more complicated it becomes to predict the membrane potential. However, this can be done using the Goldman-Hodgkin-Katz equation or the weighted means equation. By plugging in the concentration gradients and the permeabilities of the ions at any instant in time, one can determine the membrane potential at that moment. What the GHK equations means is that, at any time, the value of the membrane potential will be a weighted average of the equilibrium potentials of all permeant ions. The "weighting" is the ions relative permeability across the membrane.
离子越多,预测膜电位就越复杂。然而,这可以用 Goldman-Hodgkin-Katz 方程或加权平均数方程来实现。通过在任意时刻输入离子的浓度梯度和渗透率,就可以在那个时刻测定膜电位。方程的意思是,在任何时候,膜电位的值都是所有离子平衡势的加权平均数。“权重”是离子在膜上的相对渗透性。
Effects and implications 效应与意义
While cells expend energy to transport ions and establish a transmembrane potential, they use this potential in turn to transport other ions and metabolites such as sugar. The transmembrane potential of the mitochondria drives the production of ATP, which is the common currency of biological energy.
当细胞消耗能量来转运离子并建立膜电位时,它们反过来利用这种势能来转运其他离子和代谢物,如糖。线粒体的膜电位驱动 ATP 的产生,ATP 是生物能量的通货。
Cells may draw on the energy they store in the resting potential to drive action potentials or other forms of excitation. These changes in the membrane potential enable communication with other cells (as with action potentials) or initiate changes inside the cell, which happens in an egg when it is fertilized by a sperm.
细胞可以利用它们在静息电位中储存的能量来驱动动作电位或其他形式的兴奋。膜电位的这些变化可以与其他细胞交流(如动作电位),或产生在细胞内反应,比如卵细胞和精子受精时发生的。
In neuronal cells, an action potential begins with a rush of sodium ions into the cell through sodium channels, resulting in depolarization, while recovery involves an outward rush of potassium through potassium channels. Both of these fluxes occur by passive diffusion.
在神经细胞,一个动作电位开始于钠离子通过钠离子通道涌入细胞,导致去极化,而恢复过程中钾离子通过钾离子通道向外涌入。这两种流向都是通过被动扩散产生的。
See also
- Bioelectrochemistry
- Electrochemical potential
- Goldman equation
- Membrane biophysics
- Microelectrode array
- Saltatory conduction
- Surface potential
- Gibbs–Donnan effect
- Synaptic potential
Notes
- ↑ Note that the signs of ENa and EK are opposite. This is because the concentration gradient for potassium is directed out of the cell, while the concentration gradient for sodium is directed into the cell. Membrane potentials are defined relative to the exterior of the cell; thus, a potential of −70 mV implies that the interior of the cell is negative relative to the exterior.
Note that the signs of ENa and EK are opposite. This is because the concentration gradient for potassium is directed out of the cell, while the concentration gradient for sodium is directed into the cell. Membrane potentials are defined relative to the exterior of the cell; thus, a potential of −70 mV implies that the interior of the cell is negative relative to the exterior.
注意,e < sub > Na 和 e < sub > k 的符号相反。这是因为钾的浓度梯度指向细胞外,而钠的浓度梯度指向细胞内。膜电位是相对于细胞外部定义的,因此,电位 -70 mV 意味着细胞内部相对于外部是负的。
References
- ↑ Bruce, Alberts (2014-11-18). Molecular biology of the cell (Sixth ed.). New York, NY. ISBN 9780815344322. OCLC 887605755.
- ↑ Abdul Kadir, Lina; Stacey, Michael; Barrett-Jolley, Richard (2018). "Emerging Roles of the Membrane Potential: Action Beyond the Action Potential". Frontiers in Physiology (in English). 9. doi:10.3389/fphys.2018.01661. ISSN 1664-042X. PMID 30519193.
- ↑ 3.0 3.1 Campbell Biology, 6th edition
- ↑ 4.0 4.1 Johnston and Wu, p. 9.
- ↑ 5.0 5.1 5.2 5.3 Bullock, Orkand, and Grinnell, pp. 140–41.
- ↑ 6.0 6.1 Bullock, Orkand, and Grinnell, pp. 153–54.
- ↑ 7.0 7.1 Mummert H, Gradmann D (1991). "Action potentials in Acetabularia: measurement and simulation of voltage-gated fluxes". Journal of Membrane Biology. 124 (3): 265–73. doi:10.1007/BF01994359. PMID 1664861. S2CID 22063907.
- ↑ 8.0 8.1 Schmidt-Nielsen, p. 483.
- ↑ 9.0 9.1 "Chapter 2. Simple Diffusion across the Membrane Barrier". Transport and Diffusion across Cell Membranes. San Diego: Academic Press. 1986. pp. 69–112. ISBN 978-0-12-664661-0.
- ↑ 10.0 10.1 10.2 10.3 Hodgkin AL, Keynes RD (1955). "Active transport of cations in giant axons from Sepia and Loligo". J. Physiol. 128 (1): 28–60. doi:10.1113/jphysiol.1955.sp005290. PMC 1365754. PMID 14368574.
- ↑ 11.0 11.1 Caldwell PC, Hodgkin AL, Keynes RD, Shaw TI (1960). "The effects of injecting energy-rich phosphate compounds on the active transport of ions in the giant axons of Loligo". J. Physiol. 152 (3): 561–90. doi:10.1113/jphysiol.1960.sp006509. PMC 1363339. PMID 13806926.
- ↑ 12.0 12.1 Steinbach HB, Spiegelman S (1943). "The sodium and potassium balance in squid nerve axoplasm". J. Cell. Comp. Physiol. 22 (2): 187–96. doi:10.1002/jcp.1030220209.
- ↑ 13.0 13.1 13.2 13.3 Hodgkin AL (1951). "The ionic basis of electrical activity in nerve and muscle". Biol. Rev. 26 (4): 339–409. doi:10.1111/j.1469-185X.1951.tb01204.x. S2CID 86282580.
- ↑ 14.0 14.1 CRC Handbook of Chemistry and Physics, 83rd edition, , pp. 12–14 to 12–16.
- ↑ 15.0 15.1 Eisenman G (1961). "On the elementary atomic origin of equilibrium ionic specificity". In A Kleinzeller. Symposium on Membrane Transport and Metabolism. New York: Academic Press. pp. 163–79.Eisenman G (1965). "Some elementary factors involved in specific ion permeation". Proc. 23rd Int. Congr. Physiol. Sci., Tokyo. Amsterdam: Excerta Med. Found.. pp. 489–506.
* Diamond JM, Wright EM (1969). "Biological membranes: the physical basis of ion and nonekectrolyte selectivity". Annual Review of Physiology. 31: 581–646. doi:10.1146/annurev.ph.31.030169.003053. PMID 4885777. - ↑ 16.0 16.1 Junge, pp. 33–37.
- ↑ Cai SQ, Li W, Sesti F (2007). "Multiple modes of a-type potassium current regulation". Curr. Pharm. Des. 13 (31): 3178–84. doi:10.2174/138161207782341286. PMID 18045167.
- ↑ 18.0 18.1 Goldin AL (2007). "Neuronal Channels and Receptors". In Waxman SG. Molecular Neurology. Burlington, MA: Elsevier Academic Press. pp. 43–58. ISBN 978-0-12-369509-3.
- ↑ 19.0 19.1 Purves et al., pp. 28–32; Bullock, Orkand, and Grinnell, pp. 133–134; Schmidt-Nielsen, pp. 478–480, 596–597; Junge, pp. 33–35
- ↑ 20.0 20.1 Sanes, Dan H.; Takács, Catherine (1993-06-01). "Activity-dependent Refinement of Inhibitory Connections". European Journal of Neuroscience (in English). 5 (6): 570–574. doi:10.1111/j.1460-9568.1993.tb00522.x. ISSN 1460-9568. PMID 8261131. S2CID 30714579.
- ↑ 21.0 21.1 KOFUJI, P.; NEWMAN, E. A. (2004-01-01). "Potassium buffering in the central nervous system". Neuroscience. 129 (4): 1045–1056. doi:10.1016/j.neuroscience.2004.06.008. ISSN 0306-4522. PMC 2322935. PMID 15561419.
- ↑ 22.0 22.1 Sanes, Dan H.; Reh, Thomas A (2012-01-01). Development of the nervous system (Third ed.). Elsevier Academic Press. pp. 211–214. ISBN 9780080923208. OCLC 762720374.
- ↑ Tosti, Elisabetta (2010-06-28). "Dynamic roles of ion currents in early development". Molecular Reproduction and Development. 77 (10): 856–867. doi:10.1002/mrd.21215. ISSN 1040-452X. PMID 20586098. S2CID 38314187.
- ↑ Boyet, M.R.; Jewell, B.R. (1981). "Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart". Progress in Biophysics and Molecular Biology. 36 (1): 1–52. doi:10.1016/0079-6107(81)90003-1. ISSN 0079-6107. PMID 7001542.
- ↑ Spinelli, Valentina; Sartiani, Laura; Mugelli, Alessandro; Romanelli, Maria Novella; Cerbai, Elisabetta (2018). "Hyperpolarization-activated cyclic-nucleotide-gated channels: pathophysiological, developmental, and pharmacological insights into their function in cellular excitability". Canadian Journal of Physiology and Pharmacology. 96 (10): 977–984. doi:10.1139/cjpp-2018-0115. hdl:1807/90084. ISSN 0008-4212. PMID 29969572.
- ↑ Jones, Brian L.; Smith, Stephen M. (2016-03-30). "Calcium-Sensing Receptor: A Key Target for Extracellular Calcium Signaling in Neurons". Frontiers in Physiology. 7: 116. doi:10.3389/fphys.2016.00116. ISSN 1664-042X. PMC 4811949. PMID 27065884.
- ↑ Debanne, Dominique; Inglebert, Yanis; Russier, Michaël (2019). "Plasticity of intrinsic neuronal excitability" (PDF). Current Opinion in Neurobiology (in English). 54: 73–82. doi:10.1016/j.conb.2018.09.001. PMID 30243042. S2CID 52812190.
- ↑ 28.0 28.1 Davenport, Bennett; Li, Yuan; Heizer, Justin W.; Schmitz, Carsten; Perraud, Anne-Laure (2015-07-23). "Signature Channels of Excitability no More: L-Type Channels in Immune Cells". Frontiers in Immunology. 6: 375. doi:10.3389/fimmu.2015.00375. ISSN 1664-3224. PMC 4512153. PMID 26257741.
- ↑ 29.0 29.1 Sakmann, Bert (2017-04-21). "From single cells and single columns to cortical networks: dendritic excitability, coincidence detection and synaptic transmission in brain slices and brains". Experimental Physiology. 102 (5): 489–521. doi:10.1113/ep085776. ISSN 0958-0670. PMC 5435930. PMID 28139019.
- ↑ 30.0 30.1 Franco R, Bortner CD, Cidlowski JA (January 2006). "Potential roles of electrogenic ion transport and plasma membrane depolarization in apoptosis". J. Membr. Biol. 209 (1): 43–58. doi:10.1007/s00232-005-0837-5. PMID 16685600. S2CID 849895.
- ↑ 31.0 31.1 Purves et al., pp. 32–33; Bullock, Orkand, and Grinnell, pp. 138–140; Schmidt-Nielsen, pp. 480; Junge, pp. 35–37
- ↑ 32.0 32.1 Spangler SG (1972). "Expansion of the constant field equation to include both divalent and monovalent ions". Alabama Journal of Medical Sciences. 9 (2): 218–23. PMID 5045041.
- ↑ 33.0 33.1 Purves et al., p. 34; Bullock, Orkand, and Grinnell, p. 134; Schmidt-Nielsen, pp. 478–480.
- ↑ 34.0 34.1 Purves et al., pp. 33–36; Bullock, Orkand, and Grinnell, p. 131.
- ↑ 35.0 35.1 35.2 35.3 Magnuson DS, Morassutti DJ, Staines WA, McBurney MW, Marshall KC (Jan 14, 1995). "In vivo electrophysiological maturation of neurons derived from a multipotent precursor (embryonal carcinoma) cell line". Developmental Brain Research. 84 (1): 130–41. doi:10.1016/0165-3806(94)00166-W. PMID 7720212.
- ↑ 36.0 36.1 Juusola M, Kouvalainen E, Järvilehto M, Weckström M (Sep 1994). "Contrast gain, signal-to-noise ratio, and linearity in light-adapted blowfly photoreceptors". J Gen Physiol. 104 (3): 593–621. doi:10.1085/jgp.104.3.593. PMC 2229225. PMID 7807062.
- ↑ 37.0 37.1 Laughlin SB, de Ruyter van Steveninck RR, Anderson JC (May 1998). "The metabolic cost of neural information". Nat. Neurosci. 1 (1): 36–41. doi:10.1038/236. PMID 10195106. S2CID 204995437.
Further reading
- Alberts et al. Molecular Biology of the Cell. Garland Publishing; 4th Bk&Cdr edition (March, 2002). Undergraduate level.
- Guyton, Arthur C., John E. Hall. Textbook of medical physiology. W.B. Saunders Company; 10th edition (August 15, 2000). Undergraduate level.
- Hille, B. Ionic Channel of Excitable Membranes Sinauer Associates, Sunderland, MA, USA; 1st Edition, 1984.
- Nicholls, J.G., Martin, A.R. and Wallace, B.G. From Neuron to Brain Sinauer Associates, Inc. Sunderland, MA, USA 3rd Edition, 1992.
- Ove-Sten Knudsen. Biological Membranes: Theory of Transport, Potentials and Electric Impulses. Cambridge University Press (September 26, 2002). Graduate level.
- National Medical Series for Independent Study. Physiology. Lippincott Williams & Wilkins. Philadelphia, PA, USA 4th Edition, 2001.
= 深入阅读 =
- Alberts et al. 。细胞分子生物学。加兰德出版社; 第4 Bk & Cdr 版(2002年3月)。.本科水平。
- 盖顿,阿瑟 · c · 约翰 · e · 霍尔。医学生理学教科书。西班牙。桑德斯公司; 第10版(2000年8月15日)。.本科水平。
- Hille,b. 兴奋膜离子通道 Sinauer Associates,Sunderland,MA,USA; 第一版,1984。
- Nicholls,j.g. ,Martin,a.r.还有华莱士,b.g。从 Neuron 到 Brain Sinauer Associates,inc. . Sunderland,MA,USA 3 rd Edition,1992。
- Ove-Sten Knudsen.生物膜: 传递、电位和电脉冲理论。剑桥大学出版社(2002年9月26日)。.研究生水平。
- 国家自主研究医学系列。生理学。利平科特 · 威廉姆斯 & 威尔金斯。费城,宾夕法尼亚州,美国第四版,2001年。
External links
- Functions of the Cell Membrane
- Nernst/Goldman Equation Simulator
- Nernst Equation Calculator
- Goldman-Hodgkin-Katz Equation Calculator
- Electrochemical Driving Force Calculator
- The Origin of the Resting Membrane Potential - Online interactive tutorial (Flash)
- Functions of the Cell Membrane
- Nernst/Goldman Equation Simulator
- Nernst Equation Calculator
- Goldman-Hodgkin-Katz Equation Calculator
- Electrochemical Driving Force Calculator
- The Origin of the Resting Membrane Potential - Online interactive tutorial (Flash)
This page was moved from wikipedia:en:Membrane potential. Its edit history can be viewed at 膜电位/edithistory